| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233814 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 9 Pages |
•Preparation and characterization of binuclear metal complexes.•Elemental analyses, IR, NMR, and molar conductance for complexes were performed.•Solid reflectance and thermal analyses (TG, DTG and DTA) have been reported.•Spectral data showed an octahedral geometry for the studied complexes.•DFT calculations investigate the equilibrium geometry of the ligand and complexes.
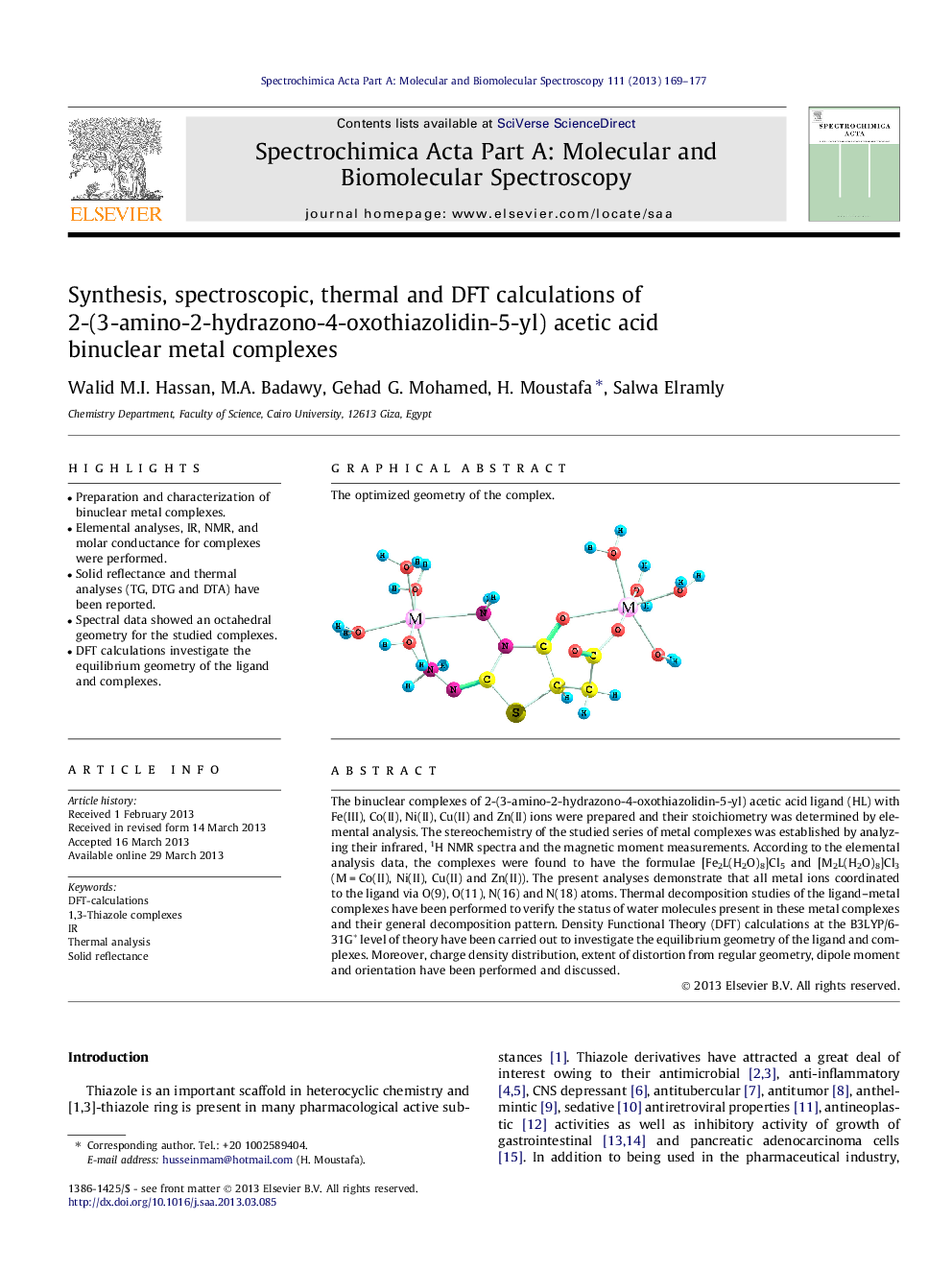
The binuclear complexes of 2-(3-amino-2-hydrazono-4-oxothiazolidin-5-yl) acetic acid ligand (HL) with Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) ions were prepared and their stoichiometry was determined by elemental analysis. The stereochemistry of the studied series of metal complexes was established by analyzing their infrared, 1H NMR spectra and the magnetic moment measurements. According to the elemental analysis data, the complexes were found to have the formulae [Fe2L(H2O)8]Cl5 and [M2L(H2O)8]Cl3 (M = Co(II), Ni(II), Cu(II) and Zn(II)). The present analyses demonstrate that all metal ions coordinated to the ligand via O(9), O(11), N(16) and N(18) atoms. Thermal decomposition studies of the ligand–metal complexes have been performed to verify the status of water molecules present in these metal complexes and their general decomposition pattern. Density Functional Theory (DFT) calculations at the B3LYP/6-31G* level of theory have been carried out to investigate the equilibrium geometry of the ligand and complexes. Moreover, charge density distribution, extent of distortion from regular geometry, dipole moment and orientation have been performed and discussed.
Graphical abstractThe optimized geometry of the complex.Figure optionsDownload full-size imageDownload as PowerPoint slide