| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233951 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Synthesis and characterization of 3-(1,3-benzothiazol-2-yl)-2-hydroxynaphthalene-1-carbaldehyde 4.•Incorporation of benzothiazole ring at the 3-position in naphthalene ring leads to solid state fluorescence.•Existence of ESIPT has been theoretically predicted by computational method.•Photophysical properties have been studied experimentally and correlated with computational results.
Excited state intramolecular proton transfer inspired 3-(1,3-benzothiazol-2-yl)-2-hydroxynaphthalene-1-carbaldehyde, showing solid state fluorescence has been synthesized. Existence of excited state intramolecular proton transfer process between carbonyl group and phenolic OH group has been theoretically predicted using computational method. Density functional theory and time dependent density functional theory computations have been used to investigate structural parameters and understand the photophysical properties of the synthesized carbaldehyde. The photophysical properties of carbaldehyde were evaluated using UV–Visible and fluorescence spectroscopy and are found to be very sensitive to the microenvironment such as solvent polarity and pH. The experimental absorption–emission a results are correlated with the computed values. The increase in the dipole moment of A2-Keto* than A2-Enol* suggested the presence of keto form in the excited state and which is responsible for the single fluorescence emission with a large Stokes shift in all solvents.
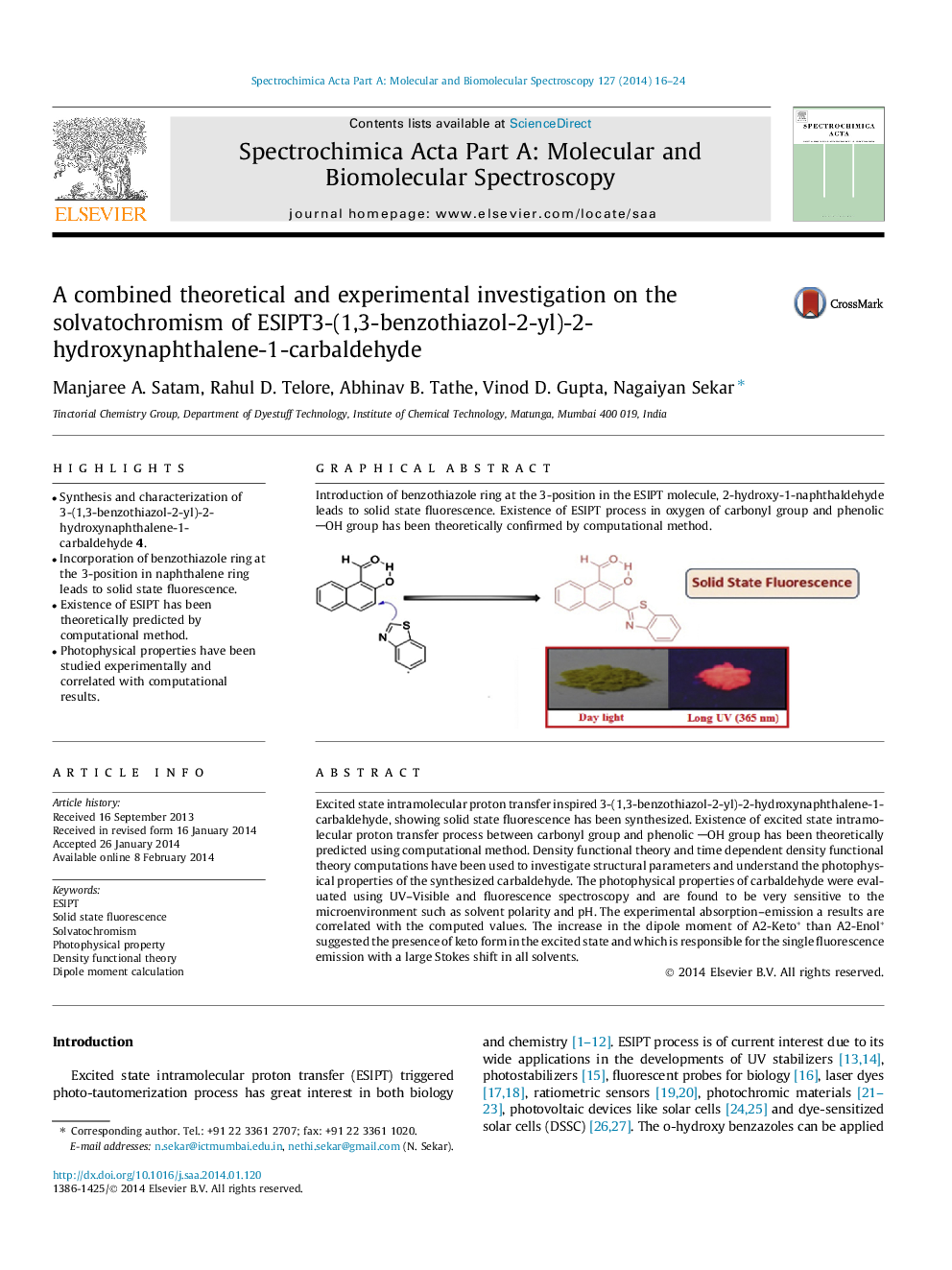
Graphical abstractIntroduction of benzothiazole ring at the 3-position in the ESIPT molecule, 2-hydroxy-1-naphthaldehyde leads to solid state fluorescence. Existence of ESIPT process in oxygen of carbonyl group and phenolic OH group has been theoretically confirmed by computational method.Figure optionsDownload full-size imageDownload as PowerPoint slide