| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233954 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
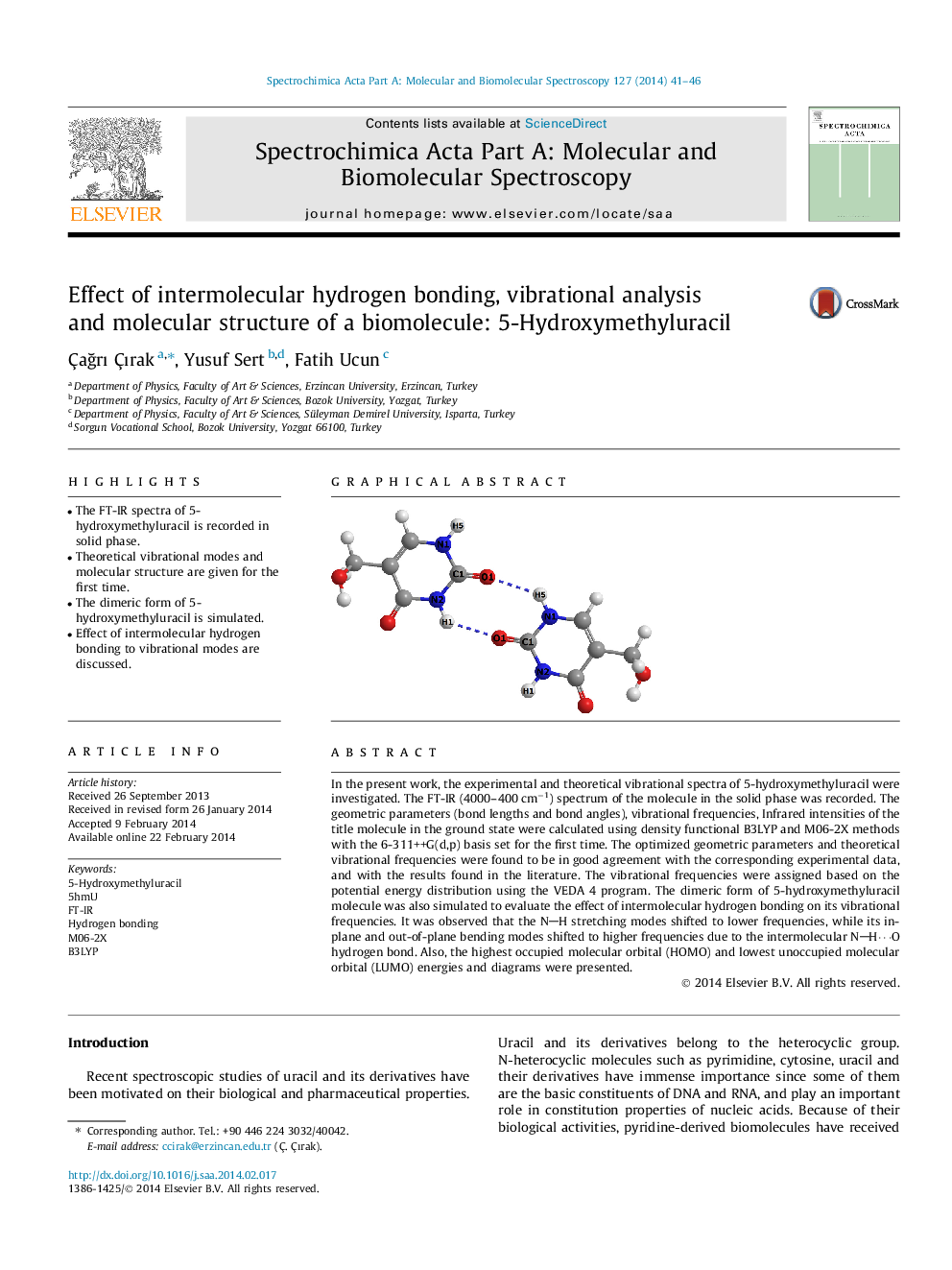
•The FT-IR spectra of 5-hydroxymethyluracil is recorded in solid phase.•Theoretical vibrational modes and molecular structure are given for the first time.•The dimeric form of 5-hydroxymethyluracil is simulated.•Effect of intermolecular hydrogen bonding to vibrational modes are discussed.
In the present work, the experimental and theoretical vibrational spectra of 5-hydroxymethyluracil were investigated. The FT-IR (4000–400 cm−1) spectrum of the molecule in the solid phase was recorded. The geometric parameters (bond lengths and bond angles), vibrational frequencies, Infrared intensities of the title molecule in the ground state were calculated using density functional B3LYP and M06-2X methods with the 6-311++G(d,p) basis set for the first time. The optimized geometric parameters and theoretical vibrational frequencies were found to be in good agreement with the corresponding experimental data, and with the results found in the literature. The vibrational frequencies were assigned based on the potential energy distribution using the VEDA 4 program. The dimeric form of 5-hydroxymethyluracil molecule was also simulated to evaluate the effect of intermolecular hydrogen bonding on its vibrational frequencies. It was observed that the NH stretching modes shifted to lower frequencies, while its in-plane and out-of-plane bending modes shifted to higher frequencies due to the intermolecular NH⋯O hydrogen bond. Also, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies and diagrams were presented.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide