| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233967 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•Synthesizing two zinc complexes constructed from indoleacetic acid.•Discussing the UV–vis absorption spectra.•Discussing the photoluminescent properties of two complexes.
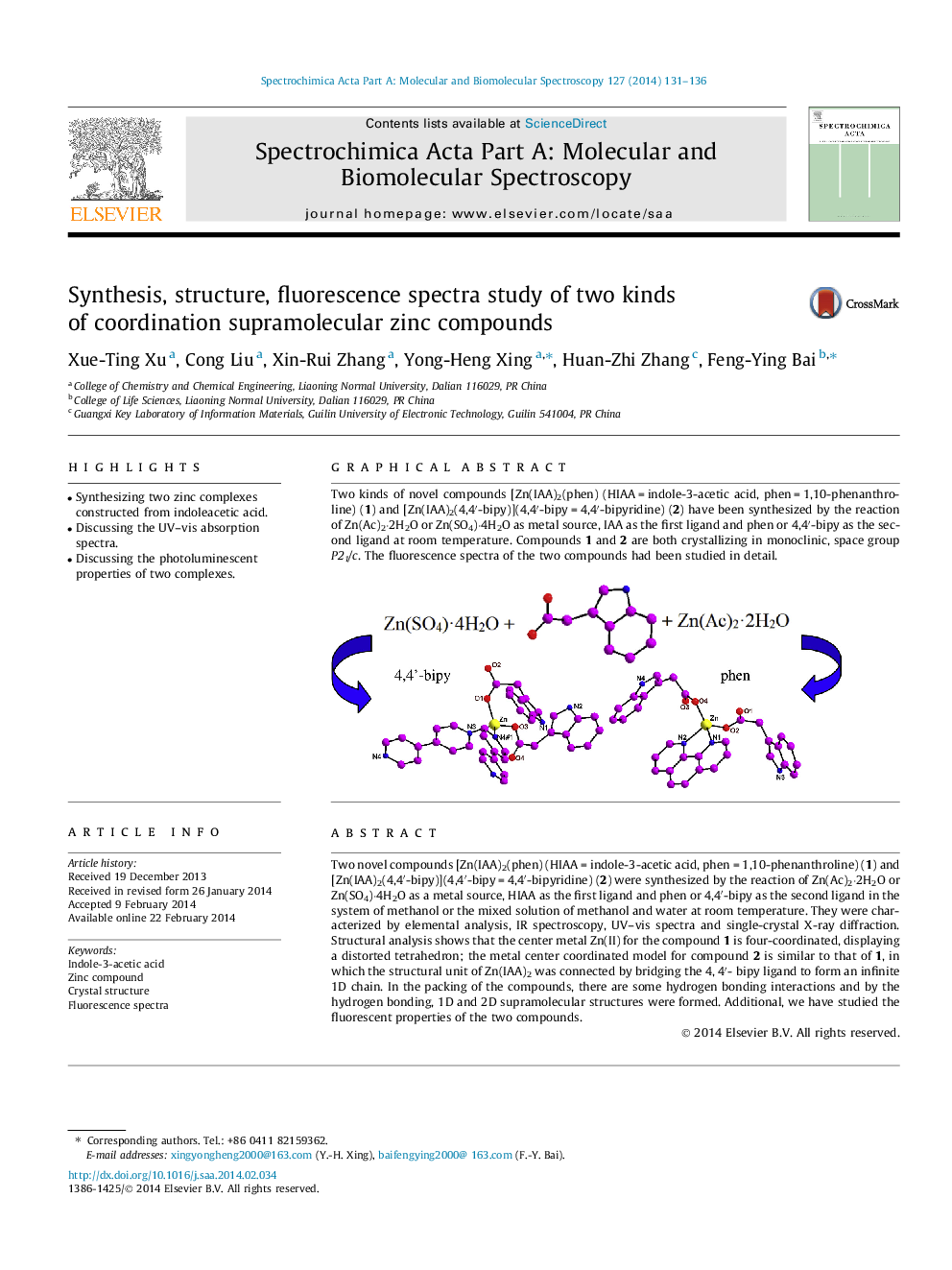
Two novel compounds [Zn(IAA)2(phen) (HIAA = indole-3-acetic acid, phen = 1,10-phenanthroline) (1) and [Zn(IAA)2(4,4′-bipy)](4,4′-bipy = 4,4′-bipyridine) (2) were synthesized by the reaction of Zn(Ac)2·2H2O or Zn(SO4)·4H2O as a metal source, HIAA as the first ligand and phen or 4,4′-bipy as the second ligand in the system of methanol or the mixed solution of methanol and water at room temperature. They were characterized by elemental analysis, IR spectroscopy, UV–vis spectra and single-crystal X-ray diffraction. Structural analysis shows that the center metal Zn(II) for the compound 1 is four-coordinated, displaying a distorted tetrahedron; the metal center coordinated model for compound 2 is similar to that of 1, in which the structural unit of Zn(IAA)2 was connected by bridging the 4, 4′- bipy ligand to form an infinite 1D chain. In the packing of the compounds, there are some hydrogen bonding interactions and by the hydrogen bonding, 1D and 2D supramolecular structures were formed. Additional, we have studied the fluorescent properties of the two compounds.
Graphical abstractTwo kinds of novel compounds [Zn(IAA)2(phen) (HIAA = indole-3-acetic acid, phen = 1,10-phenanthroline) (1) and [Zn(IAA)2(4,4′-bipy)](4,4′-bipy = 4,4′-bipyridine) (2) have been synthesized by the reaction of Zn(Ac)2·2H2O or Zn(SO4)·4H2O as metal source, IAA as the first ligand and phen or 4,4′-bipy as the second ligand at room temperature. Compounds 1 and 2 are both crystallizing in monoclinic, space group P21/c. The fluorescence spectra of the two compounds had been studied in detail.Figure optionsDownload full-size imageDownload as PowerPoint slide