| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233985 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
•The complete assignments of 2,5-DACM were performed on the basis of PED.•Nonlinear optical properties of 2,5-DACM were studied using DFT calculations.•HOMO–LUMO energy gap explains the charge transfer interactions in the molecule.•Stability of the molecule has been analyzed using NBO analysis.
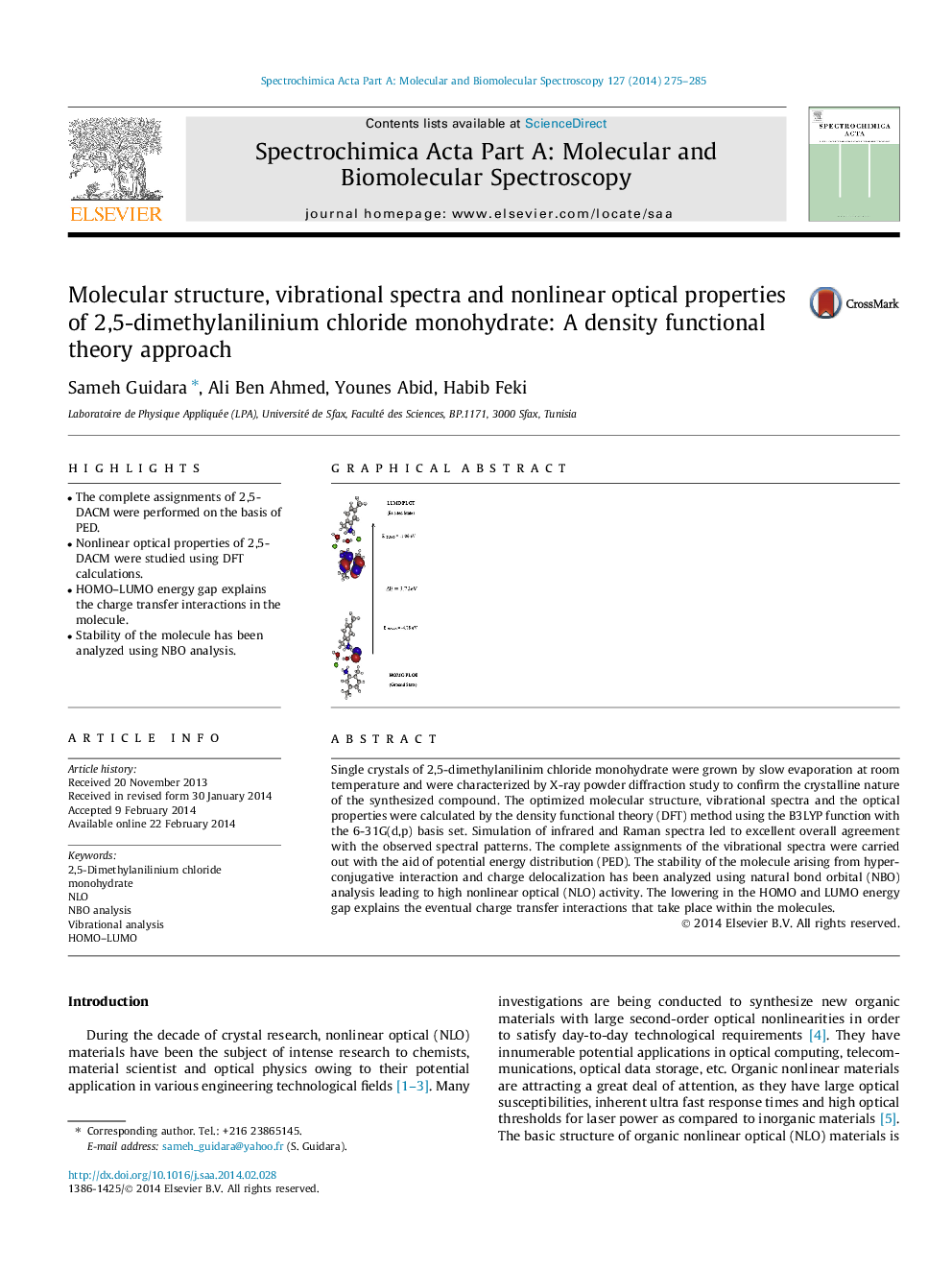
Single crystals of 2,5-dimethylanilinim chloride monohydrate were grown by slow evaporation at room temperature and were characterized by X-ray powder diffraction study to confirm the crystalline nature of the synthesized compound. The optimized molecular structure, vibrational spectra and the optical properties were calculated by the density functional theory (DFT) method using the B3LYP function with the 6-31G(d,p) basis set. Simulation of infrared and Raman spectra led to excellent overall agreement with the observed spectral patterns. The complete assignments of the vibrational spectra were carried out with the aid of potential energy distribution (PED). The stability of the molecule arising from hyperconjugative interaction and charge delocalization has been analyzed using natural bond orbital (NBO) analysis leading to high nonlinear optical (NLO) activity. The lowering in the HOMO and LUMO energy gap explains the eventual charge transfer interactions that take place within the molecules.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide