| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233992 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
·Specific interactions in polar solvents are main interactions in solvatochromism of Sudan.·GICT, LICT and sterically hindered control Sudan dyes spectroscopic behavior.·Sudan black B has highest charge individuation upon excitation in comparison to SudanIII and IV.
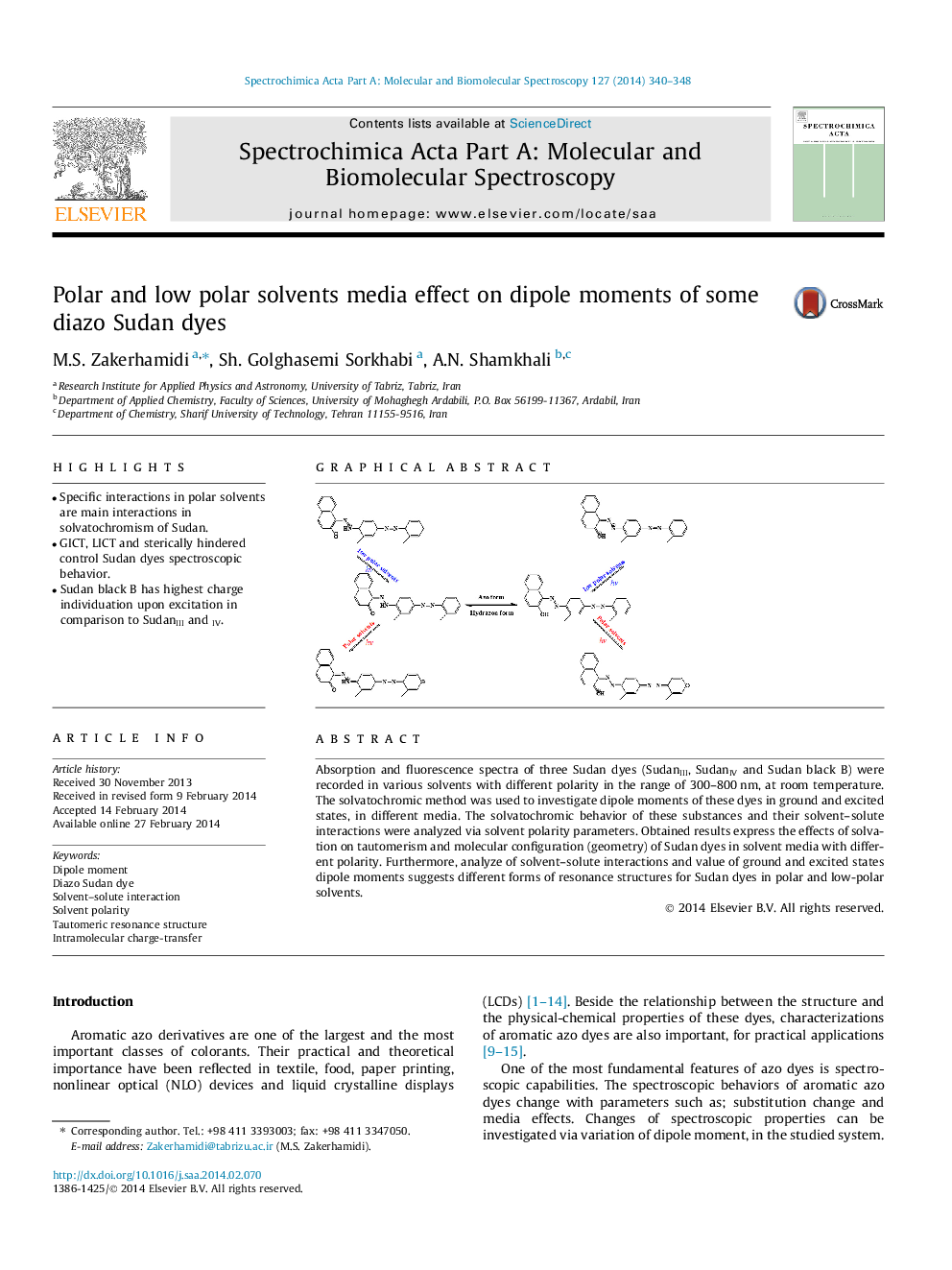
Absorption and fluorescence spectra of three Sudan dyes (SudanIII, SudanIV and Sudan black B) were recorded in various solvents with different polarity in the range of 300–800 nm, at room temperature. The solvatochromic method was used to investigate dipole moments of these dyes in ground and excited states, in different media. The solvatochromic behavior of these substances and their solvent–solute interactions were analyzed via solvent polarity parameters. Obtained results express the effects of solvation on tautomerism and molecular configuration (geometry) of Sudan dyes in solvent media with different polarity. Furthermore, analyze of solvent–solute interactions and value of ground and excited states dipole moments suggests different forms of resonance structures for Sudan dyes in polar and low-polar solvents.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide