| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234102 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
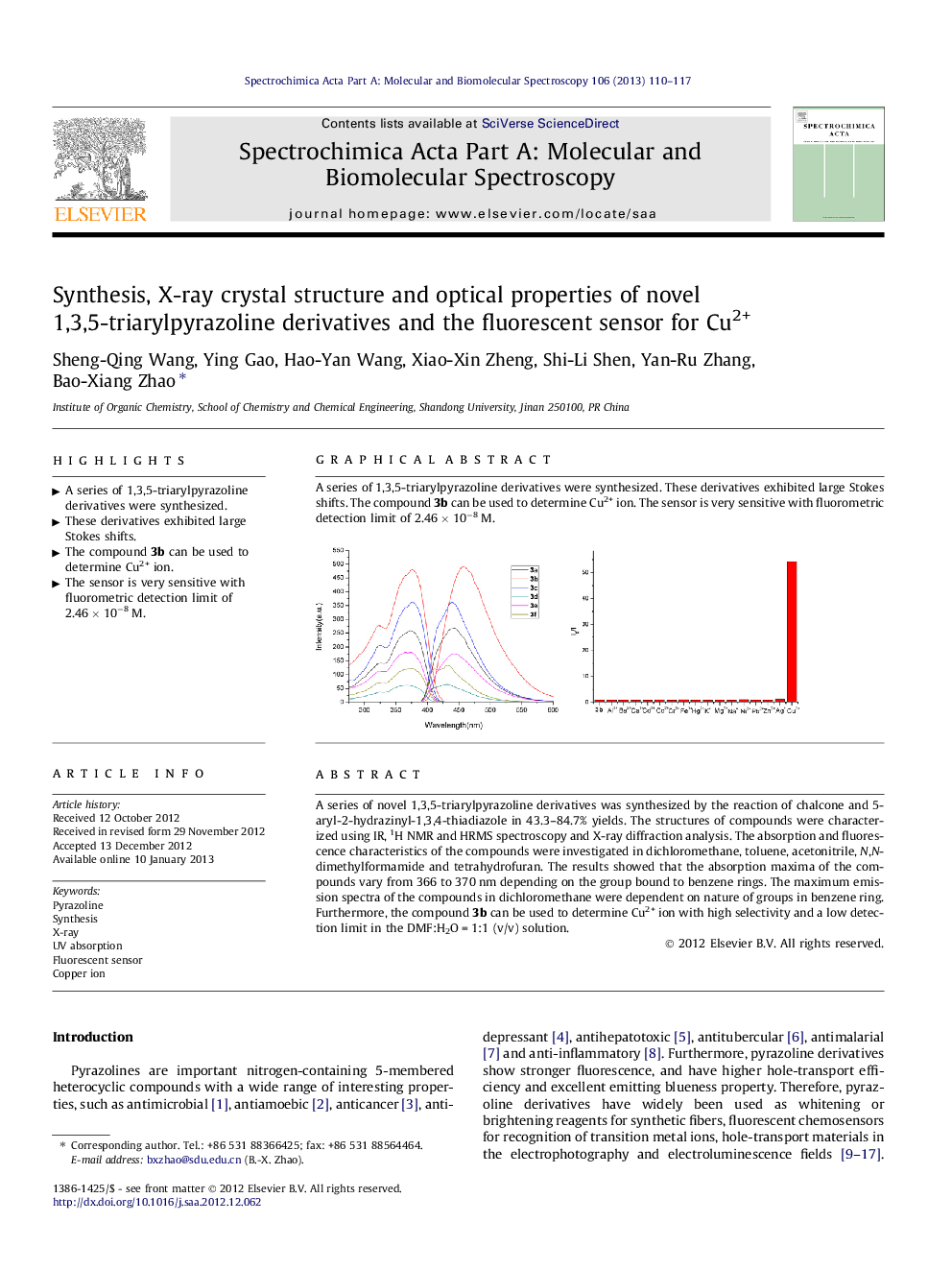
A series of novel 1,3,5-triarylpyrazoline derivatives was synthesized by the reaction of chalcone and 5-aryl-2-hydrazinyl-1,3,4-thiadiazole in 43.3–84.7% yields. The structures of compounds were characterized using IR, 1H NMR and HRMS spectroscopy and X-ray diffraction analysis. The absorption and fluorescence characteristics of the compounds were investigated in dichloromethane, toluene, acetonitrile, N,N-dimethylformamide and tetrahydrofuran. The results showed that the absorption maxima of the compounds vary from 366 to 370 nm depending on the group bound to benzene rings. The maximum emission spectra of the compounds in dichloromethane were dependent on nature of groups in benzene ring. Furthermore, the compound 3b can be used to determine Cu2+ ion with high selectivity and a low detection limit in the DMF:H2O = 1:1 (v/v) solution.
Graphical abstractA series of 1,3,5-triarylpyrazoline derivatives were synthesized. These derivatives exhibited large Stokes shifts. The compound 3b can be used to determine Cu2+ ion. The sensor is very sensitive with fluorometric detection limit of 2.46 × 10−8 M.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► A series of 1,3,5-triarylpyrazoline derivatives were synthesized. ► These derivatives exhibited large Stokes shifts. ► The compound 3b can be used to determine Cu2+ ion. ► The sensor is very sensitive with fluorometric detection limit of 2.46 × 10−8 M.