| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234114 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 7 Pages |
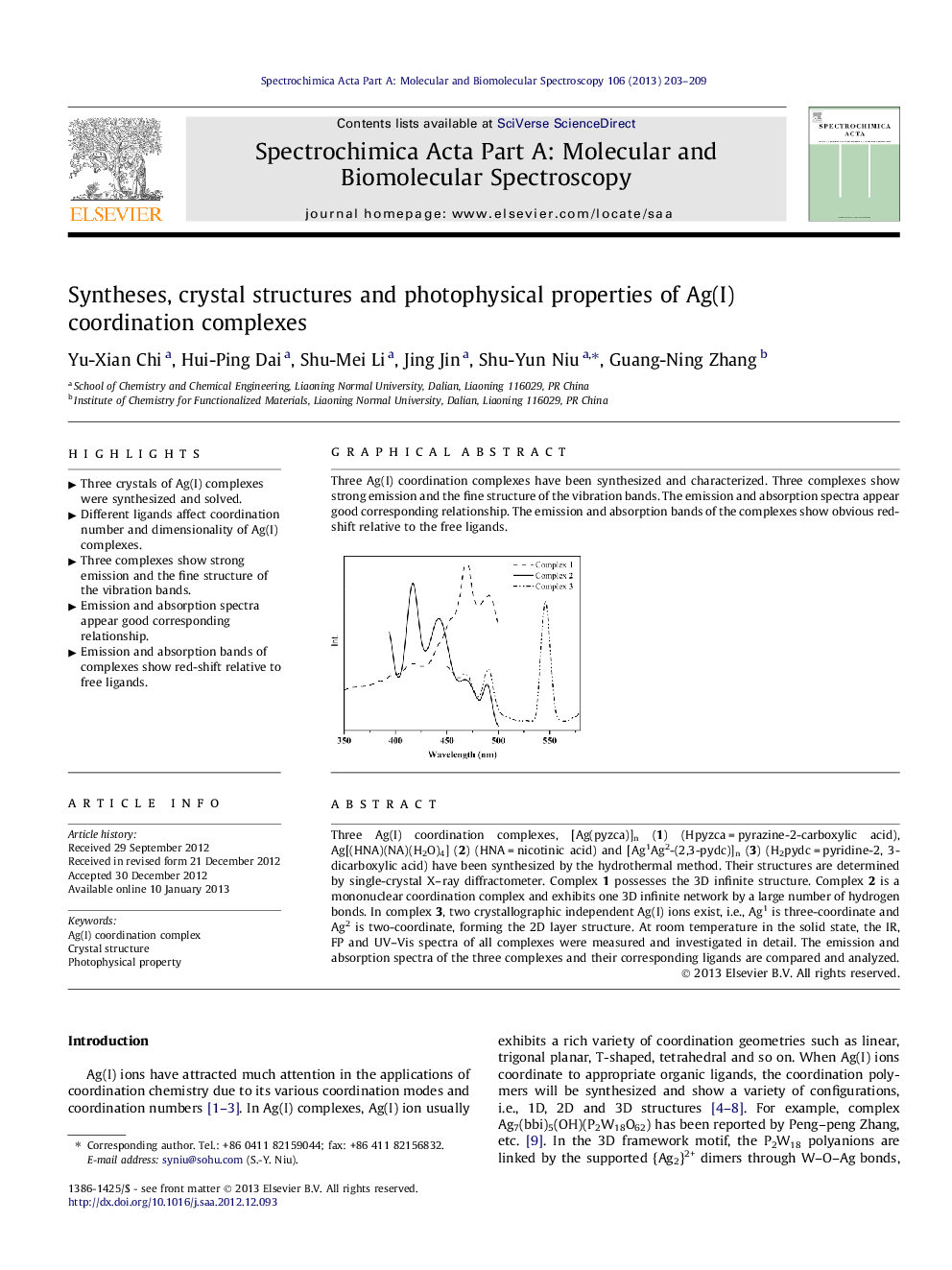
Three Ag(I) coordination complexes, [Ag(pyzca)]n (1) (Hpyzca = pyrazine-2-carboxylic acid), Ag[(HNA)(NA)(H2O)4] (2) (HNA = nicotinic acid) and [Ag1Ag2-(2,3-pydc)]n (3) (H2pydc = pyridine-2, 3-dicarboxylic acid) have been synthesized by the hydrothermal method. Their structures are determined by single-crystal X–ray diffractometer. Complex 1 possesses the 3D infinite structure. Complex 2 is a mononuclear coordination complex and exhibits one 3D infinite network by a large number of hydrogen bonds. In complex 3, two crystallographic independent Ag(I) ions exist, i.e., Ag1 is three-coordinate and Ag2 is two-coordinate, forming the 2D layer structure. At room temperature in the solid state, the IR, FP and UV–Vis spectra of all complexes were measured and investigated in detail. The emission and absorption spectra of the three complexes and their corresponding ligands are compared and analyzed.
Graphical abstractThree Ag(I) coordination complexes have been synthesized and characterized. Three complexes show strong emission and the fine structure of the vibration bands. The emission and absorption spectra appear good corresponding relationship. The emission and absorption bands of the complexes show obvious red-shift relative to the free ligands.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Three crystals of Ag(I) complexes were synthesized and solved. ► Different ligands affect coordination number and dimensionality of Ag(I) complexes. ► Three complexes show strong emission and the fine structure of the vibration bands. ► Emission and absorption spectra appear good corresponding relationship. ► Emission and absorption bands of complexes show red-shift relative to free ligands.