| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234261 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
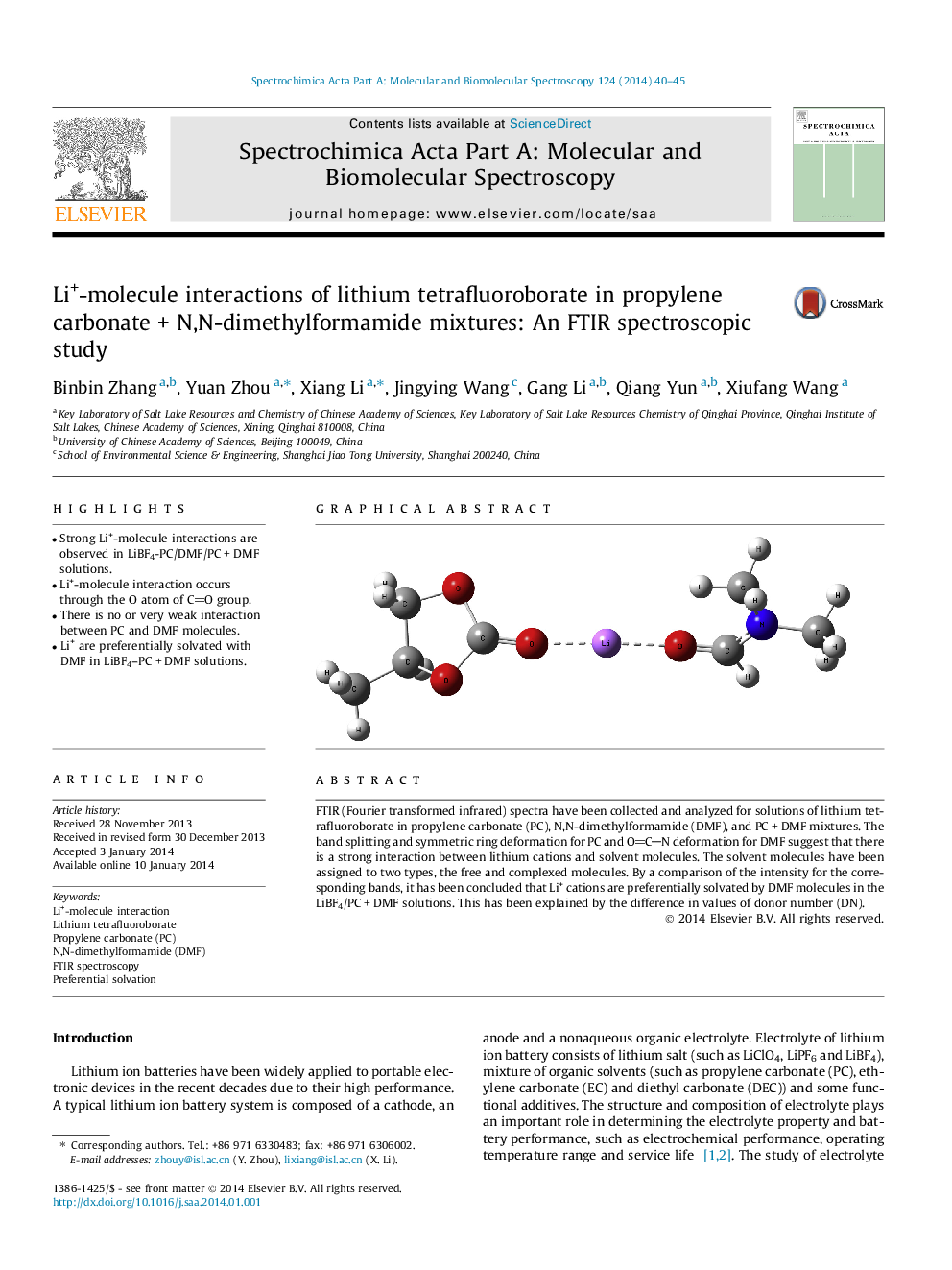
•Strong Li+-molecule interactions are observed in LiBF4-PC/DMF/PC + DMF solutions.•Li+-molecule interaction occurs through the O atom of CO group.•There is no or very weak interaction between PC and DMF molecules.•Li+ are preferentially solvated with DMF in LiBF4–PC + DMF solutions.
FTIR (Fourier transformed infrared) spectra have been collected and analyzed for solutions of lithium tetrafluoroborate in propylene carbonate (PC), N,N-dimethylformamide (DMF), and PC + DMF mixtures. The band splitting and symmetric ring deformation for PC and OCN deformation for DMF suggest that there is a strong interaction between lithium cations and solvent molecules. The solvent molecules have been assigned to two types, the free and complexed molecules. By a comparison of the intensity for the corresponding bands, it has been concluded that Li+ cations are preferentially solvated by DMF molecules in the LiBF4/PC + DMF solutions. This has been explained by the difference in values of donor number (DN).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide