| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234266 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•We use (PAN/AC) composite as adsorbent for the removal of textile dyes.•XRD and FRIR studies have been carried out for characterization of the composite.•Langmuir and Freundlich isotherms have been used to find out the best fit model.•Kinetic and thermodynamic studies of the surfactant adsorption have been investigated.•Results are of marked significance to the water treatment industries.
The adsorption of Acid Red 57 (AR57) onto Polyacrylonitrile/activated carbon (PAN/AC) composite was investigated in aqueous solution in a batch system with respect to contact time, pH and temperature. Physical characteristics of (PAN/AC) composite such as fourier transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM) were obtained. Langmuir and Freundlich adsorption models were applied to describe the equilibrium isotherms and the isotherm constants were determined. The activation energy of adsorption was also evaluated for the adsorption of AR57 onto (PAN/AC) composite. The pseudo-first-order and pseudo-second-order kinetic models were used to describe the kinetic data. The dynamic data fitted the pseudo-second-order kinetic model well. The activation energy, change of free energy, enthalpy and entropy of adsorption were also evaluated for the adsorption of AR57 onto (PAN/AC) composite. The thermodynamics of the adsorption indicated spontaneous and exothermic nature of the process. The results indicate that (PAN/AC) composite could be employed as low-cost material for the removal of acid dyes from textile effluents.
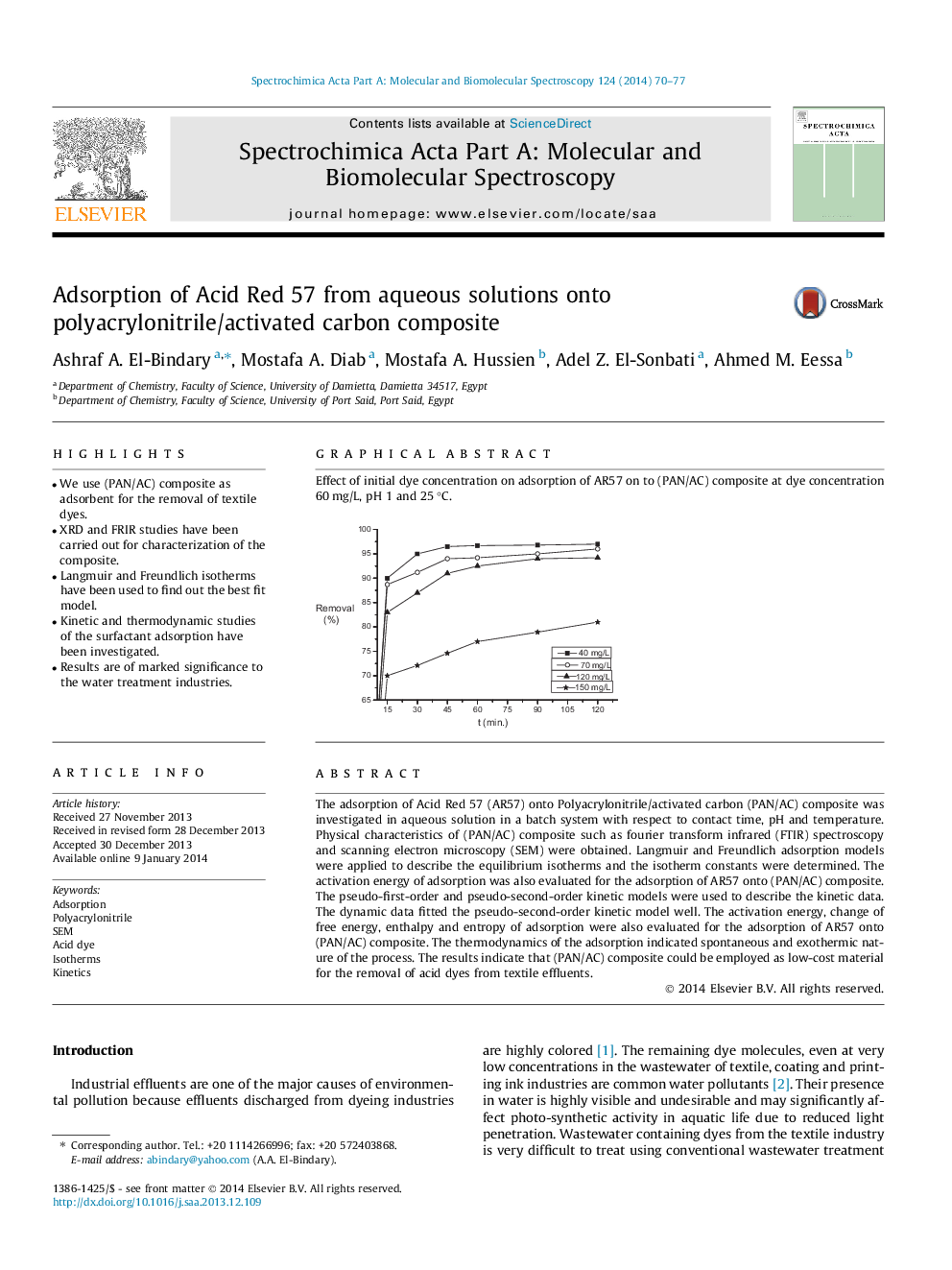
Graphical abstractEffect of initial dye concentration on adsorption of AR57 on to (PAN/AC) composite at dye concentration 60 mg/L, pH 1 and 25 °C.Figure optionsDownload full-size imageDownload as PowerPoint slide