| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234269 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•Asymmetrical 1,3,4-oxadiazole dyes were efficiently synthesized.•Structure–optical properties relationships were investigated.•The compounds exhibit bright violet–blue emission with high fluorescence quantum yields.•The high HOMO levels (−5.17 to −5.03 eV) are beneficial for hole-transporting.
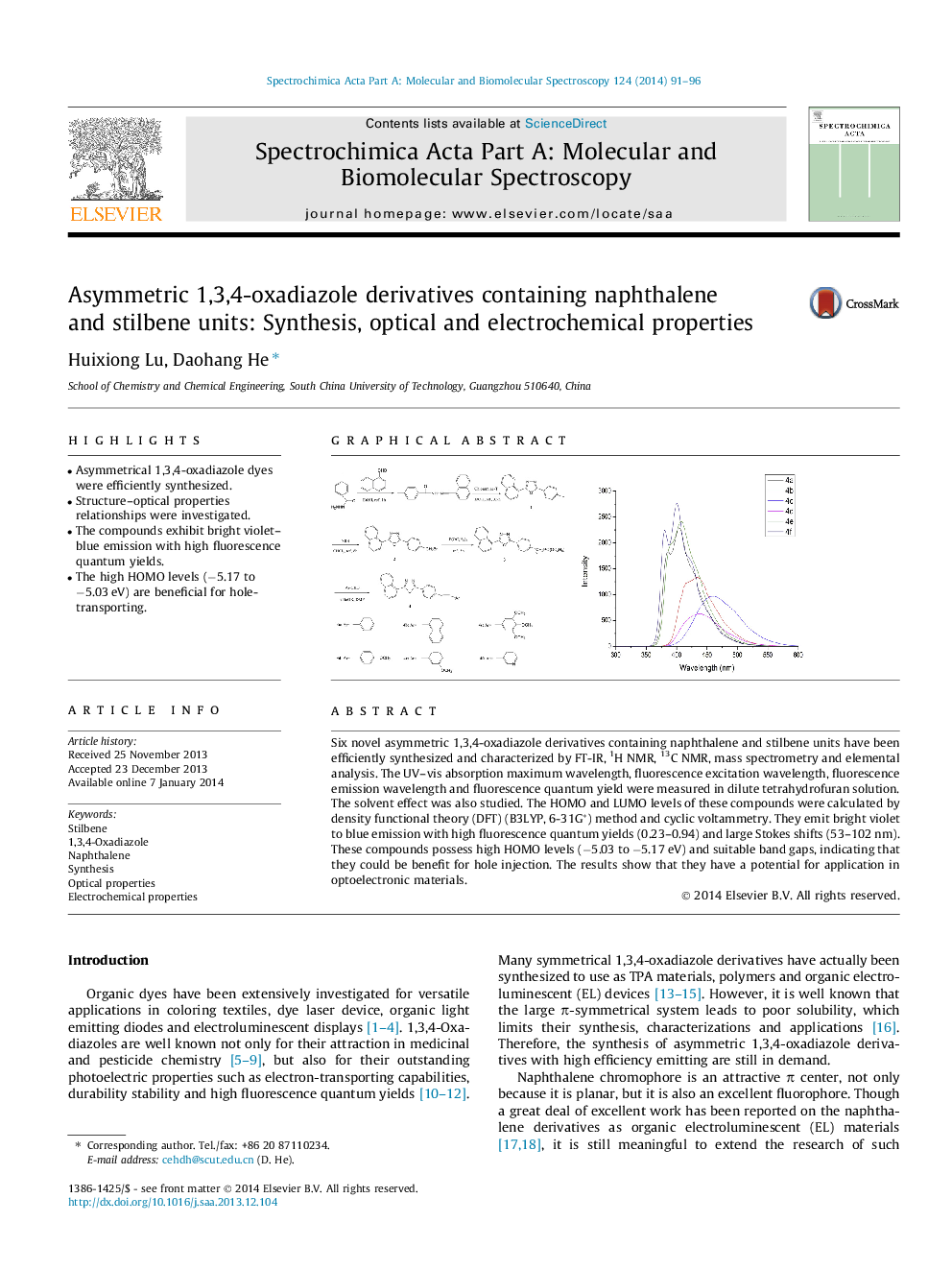
Six novel asymmetric 1,3,4-oxadiazole derivatives containing naphthalene and stilbene units have been efficiently synthesized and characterized by FT-IR, 1H NMR, 13C NMR, mass spectrometry and elemental analysis. The UV–vis absorption maximum wavelength, fluorescence excitation wavelength, fluorescence emission wavelength and fluorescence quantum yield were measured in dilute tetrahydrofuran solution. The solvent effect was also studied. The HOMO and LUMO levels of these compounds were calculated by density functional theory (DFT) (B3LYP, 6-31G*) method and cyclic voltammetry. They emit bright violet to blue emission with high fluorescence quantum yields (0.23–0.94) and large Stokes shifts (53–102 nm). These compounds possess high HOMO levels (−5.03 to −5.17 eV) and suitable band gaps, indicating that they could be benefit for hole injection. The results show that they have a potential for application in optoelectronic materials.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide