| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234271 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
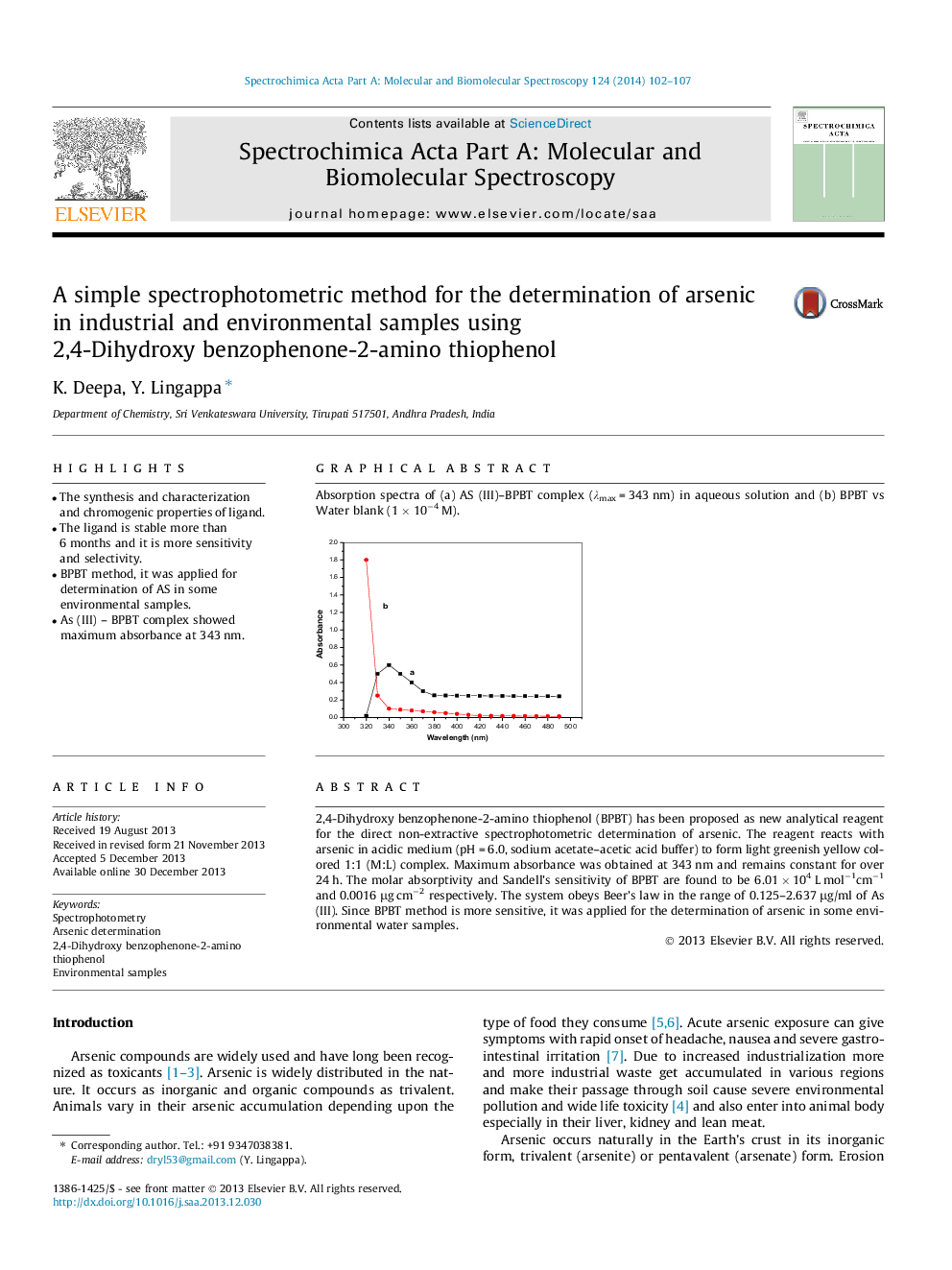
•The synthesis and characterization and chromogenic properties of ligand.•The ligand is stable more than 6 months and it is more sensitivity and selectivity.•BPBT method, it was applied for determination of AS in some environmental samples.•As (III) – BPBT complex showed maximum absorbance at 343 nm.
2,4-Dihydroxy benzophenone-2-amino thiophenol (BPBT) has been proposed as new analytical reagent for the direct non-extractive spectrophotometric determination of arsenic. The reagent reacts with arsenic in acidic medium (pH = 6.0, sodium acetate–acetic acid buffer) to form light greenish yellow colored 1:1 (M:L) complex. Maximum absorbance was obtained at 343 nm and remains constant for over 24 h. The molar absorptivity and Sandell’s sensitivity of BPBT are found to be 6.01 × 104 L mol−1cm−1 and 0.0016 μg cm−2 respectively. The system obeys Beer’s law in the range of 0.125–2.637 μg/ml of As (III). Since BPBT method is more sensitive, it was applied for the determination of arsenic in some environmental water samples.
Graphical abstractAbsorption spectra of (a) AS (III)–BPBT complex (λmax = 343 nm) in aqueous solution and (b) BPBT vs Water blank (1 × 10−4 M).Figure optionsDownload full-size imageDownload as PowerPoint slide