| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234298 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•Spectral investigation on electron transfer reactions of organic sulphides with metal ions were studied.•Biological system was stimulated with model compounds, iron(III)-bipyridyl complex and thio-diglycolic acid.•Parameters affecting the reaction, kinetics and thermodynamics were analyzed.•A model was proposed regarding the electron transfer reaction.
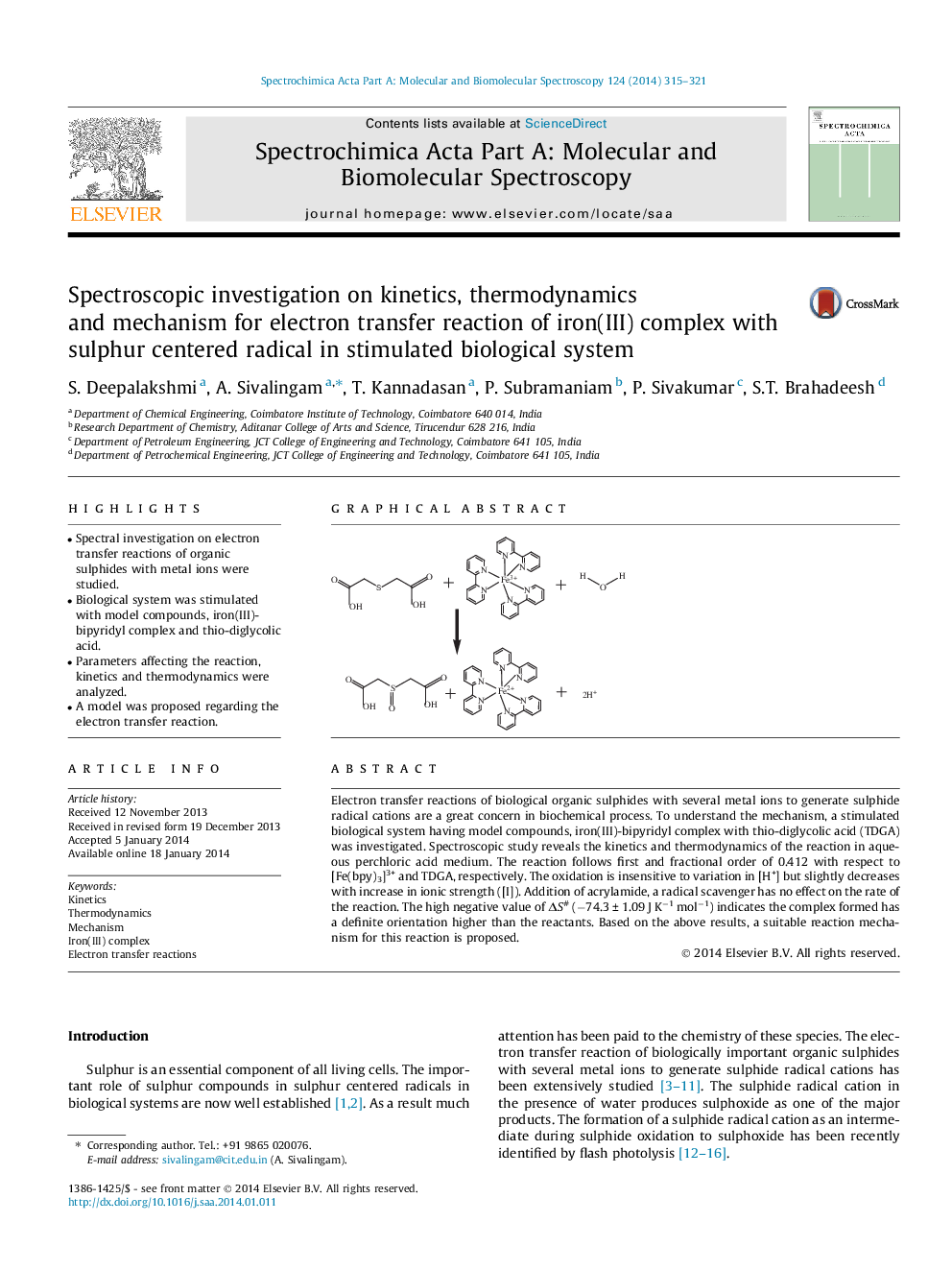
Electron transfer reactions of biological organic sulphides with several metal ions to generate sulphide radical cations are a great concern in biochemical process. To understand the mechanism, a stimulated biological system having model compounds, iron(III)-bipyridyl complex with thio-diglycolic acid (TDGA) was investigated. Spectroscopic study reveals the kinetics and thermodynamics of the reaction in aqueous perchloric acid medium. The reaction follows first and fractional order of 0.412 with respect to [Fe(bpy)3]3+ and TDGA, respectively. The oxidation is insensitive to variation in [H+] but slightly decreases with increase in ionic strength ([I]). Addition of acrylamide, a radical scavenger has no effect on the rate of the reaction. The high negative value of ΔS# (−74.3 ± 1.09 J K−1 mol−1) indicates the complex formed has a definite orientation higher than the reactants. Based on the above results, a suitable reaction mechanism for this reactionis proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide