| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234383 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 7 Pages |
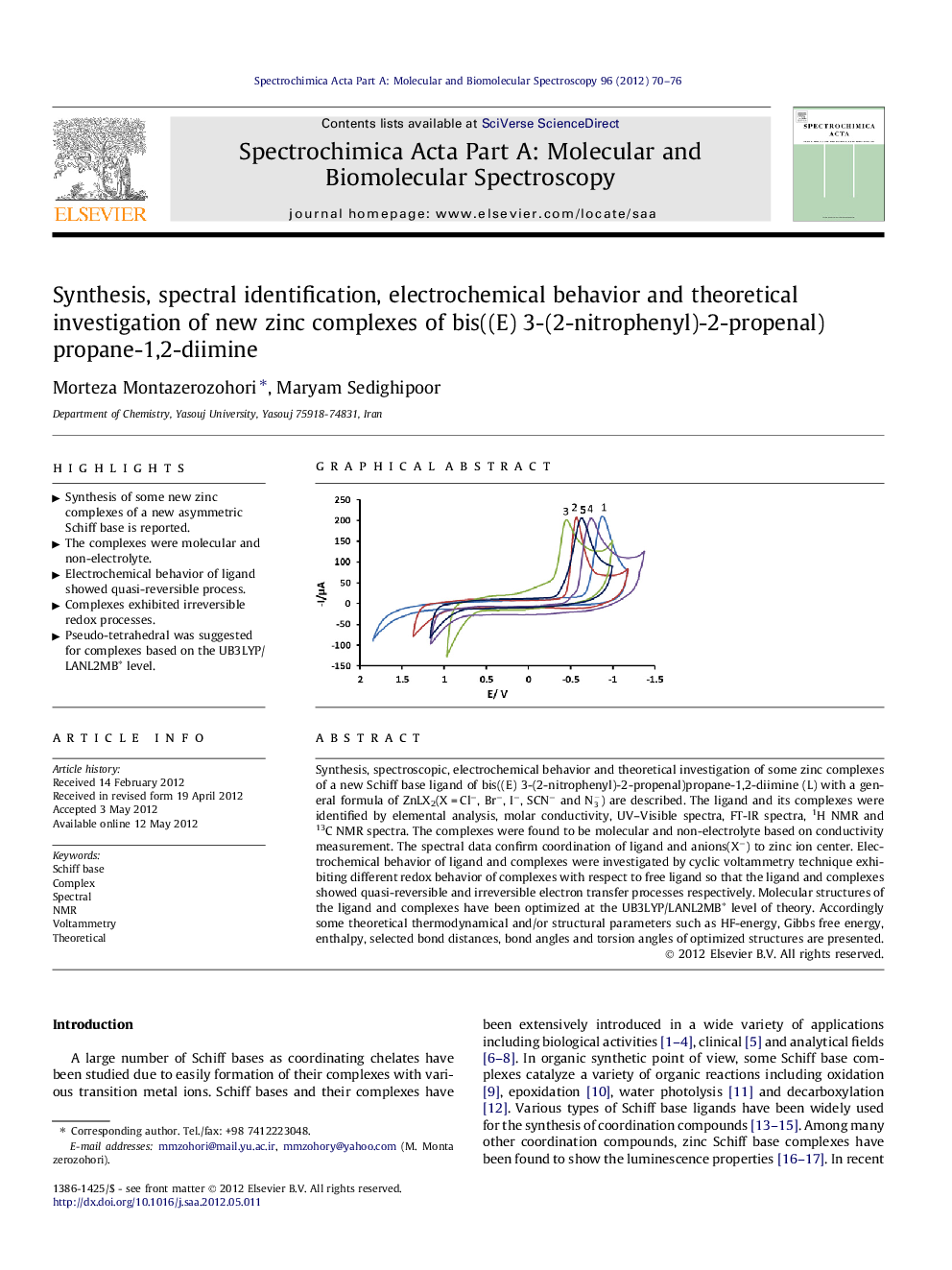
Synthesis, spectroscopic, electrochemical behavior and theoretical investigation of some zinc complexes of a new Schiff base ligand of bis((E) 3-(2-nitrophenyl)-2-propenal)propane-1,2-diimine (L) with a general formula of ZnLX2(X = Cl−, Br−, I−, SCN− and N3-) are described. The ligand and its complexes were identified by elemental analysis, molar conductivity, UV–Visible spectra, FT-IR spectra, 1H NMR and 13C NMR spectra. The complexes were found to be molecular and non-electrolyte based on conductivity measurement. The spectral data confirm coordination of ligand and anions(X−) to zinc ion center. Electrochemical behavior of ligand and complexes were investigated by cyclic voltammetry technique exhibiting different redox behavior of complexes with respect to free ligand so that the ligand and complexes showed quasi-reversible and irreversible electron transfer processes respectively. Molecular structures of the ligand and complexes have been optimized at the UB3LYP/LANL2MB∗ level of theory. Accordingly some theoretical thermodynamical and/or structural parameters such as HF-energy, Gibbs free energy, enthalpy, selected bond distances, bond angles and torsion angles of optimized structures are presented.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Synthesis of some new zinc complexes of a new asymmetric Schiff base is reported. ► The complexes were molecular and non-electrolyte. ► Electrochemical behavior of ligand showed quasi-reversible process. ► Complexes exhibited irreversible redox processes. ► Pseudo-tetrahedral was suggested for complexes based on the UB3LYP/LANL2MB∗ level.