| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234411 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 10 Pages |
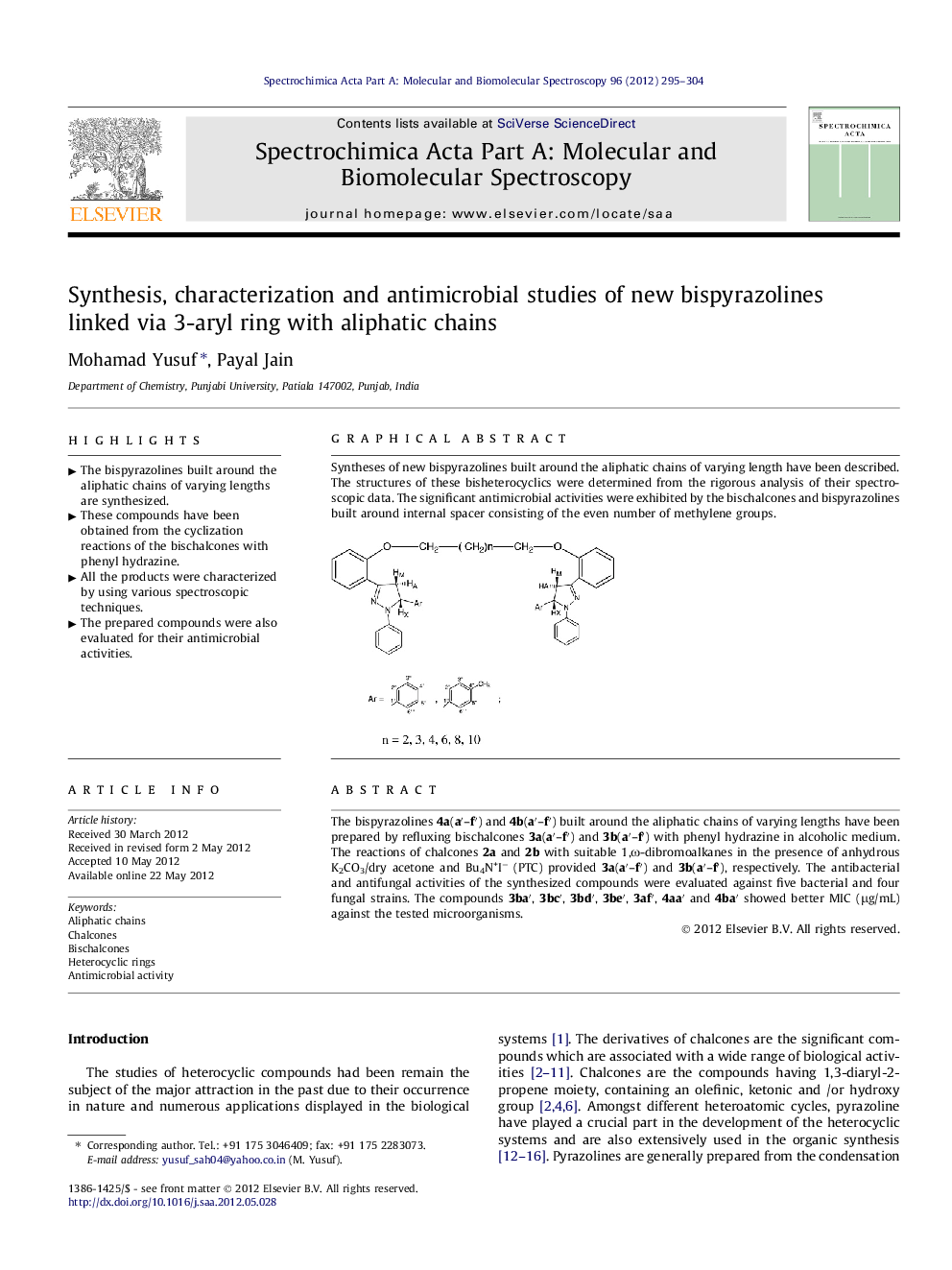
The bispyrazolines 4a(a′–f′) and 4b(a′–f′) built around the aliphatic chains of varying lengths have been prepared by refluxing bischalcones 3a(a′–f′) and 3b(a′–f′) with phenyl hydrazine in alcoholic medium. The reactions of chalcones 2a and 2b with suitable 1,ω-dibromoalkanes in the presence of anhydrous K2CO3/dry acetone and Bu4N+I− (PTC) provided 3a(a′–f′) and 3b(a′–f′), respectively. The antibacterial and antifungal activities of the synthesized compounds were evaluated against five bacterial and four fungal strains. The compounds 3ba′, 3bc′, 3bd′, 3be′, 3af′, 4aa′ and 4ba′ showed better MIC (μg/mL) against the tested microorganisms.
Graphical abstractSyntheses of new bispyrazolines built around the aliphatic chains of varying length have been described. The structures of these bisheterocyclics were determined from the rigorous analysis of their spectroscopic data. The significant antimicrobial activities were exhibited by the bischalcones and bispyrazolines built around internal spacer consisting of the even number of methylene groups.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The bispyrazolines built around the aliphatic chains of varying lengths are synthesized. ► These compounds have been obtained from the cyclization reactions of the bischalcones with phenyl hydrazine. ► All the products were characterized by using various spectroscopic techniques. ► The prepared compounds were also evaluated for their antimicrobial activities.