| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234420 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 5 Pages |
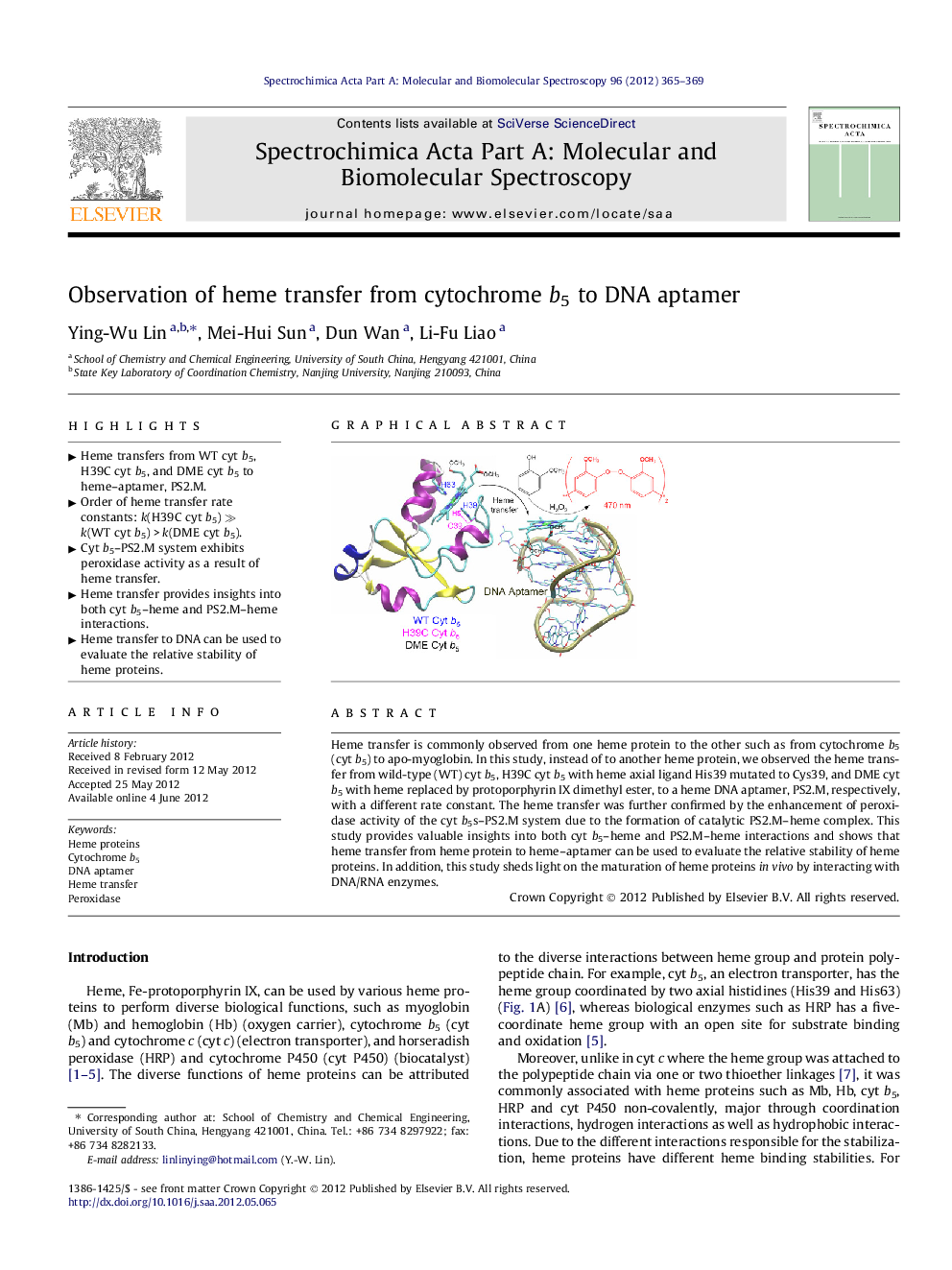
Heme transfer is commonly observed from one heme protein to the other such as from cytochrome b5 (cyt b5) to apo-myoglobin. In this study, instead of to another heme protein, we observed the heme transfer from wild-type (WT) cyt b5, H39C cyt b5 with heme axial ligand His39 mutated to Cys39, and DME cyt b5 with heme replaced by protoporphyrin IX dimethyl ester, to a heme DNA aptamer, PS2.M, respectively, with a different rate constant. The heme transfer was further confirmed by the enhancement of peroxidase activity of the cyt b5s–PS2.M system due to the formation of catalytic PS2.M–heme complex. This study provides valuable insights into both cyt b5–heme and PS2.M–heme interactions and shows that heme transfer from heme protein to heme–aptamer can be used to evaluate the relative stability of heme proteins. In addition, this study sheds light on the maturation of heme proteins in vivo by interacting with DNA/RNA enzymes.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Heme transfers from WT cyt b5, H39C cyt b5, and DME cyt b5 to heme–aptamer, PS2.M. ► Order of heme transfer rate constants: k(H39C cyt b5) ≫ k(WT cyt b5) > k(DME cyt b5). ► Cyt b5–PS2.M system exhibits peroxidase activity as a result of heme transfer. ► Heme transfer provides insights into both cyt b5–heme and PS2.M–heme interactions. ► Heme transfer to DNA can be used to evaluate the relative stability of heme proteins.