| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234427 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 15 Pages |
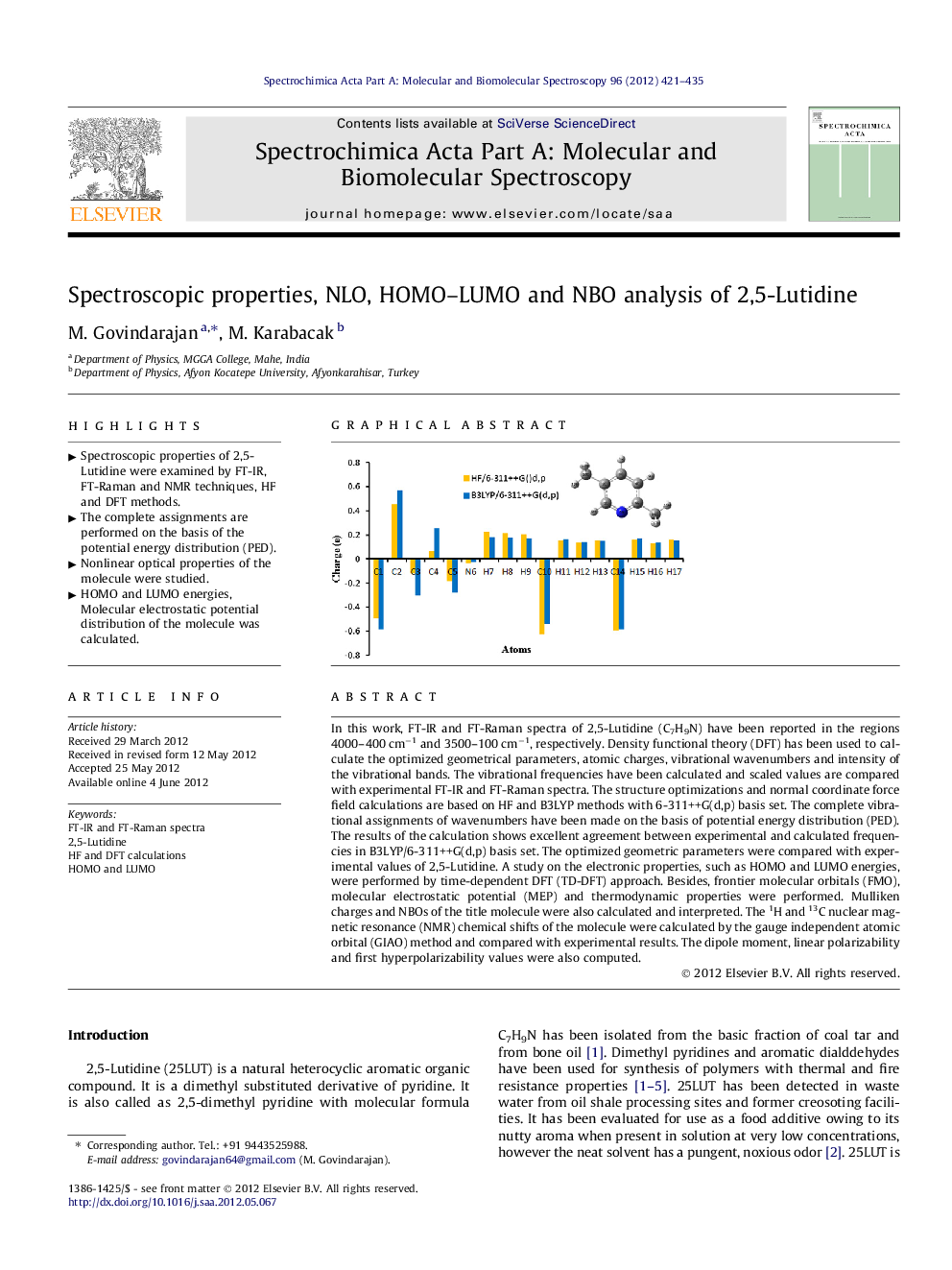
In this work, FT-IR and FT-Raman spectra of 2,5-Lutidine (C7H9N) have been reported in the regions 4000–400 cm−1 and 3500–100 cm−1, respectively. Density functional theory (DFT) has been used to calculate the optimized geometrical parameters, atomic charges, vibrational wavenumbers and intensity of the vibrational bands. The vibrational frequencies have been calculated and scaled values are compared with experimental FT-IR and FT-Raman spectra. The structure optimizations and normal coordinate force field calculations are based on HF and B3LYP methods with 6-311++G(d,p) basis set. The complete vibrational assignments of wavenumbers have been made on the basis of potential energy distribution (PED). The results of the calculation shows excellent agreement between experimental and calculated frequencies in B3LYP/6-311++G(d,p) basis set. The optimized geometric parameters were compared with experimental values of 2,5-Lutidine. A study on the electronic properties, such as HOMO and LUMO energies, were performed by time-dependent DFT (TD-DFT) approach. Besides, frontier molecular orbitals (FMO), molecular electrostatic potential (MEP) and thermodynamic properties were performed. Mulliken charges and NBOs of the title molecule were also calculated and interpreted. The 1H and 13C nuclear magnetic resonance (NMR) chemical shifts of the molecule were calculated by the gauge independent atomic orbital (GIAO) method and compared with experimental results. The dipole moment, linear polarizability and first hyperpolarizability values were also computed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Spectroscopic properties of 2,5-Lutidine were examined by FT-IR, FT-Raman and NMR techniques, HF and DFT methods. ► The complete assignments are performed on the basis of the potential energy distribution (PED). ► Nonlinear optical properties of the molecule were studied. ► HOMO and LUMO energies, Molecular electrostatic potential distribution of the molecule was calculated.