| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234431 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 7 Pages |
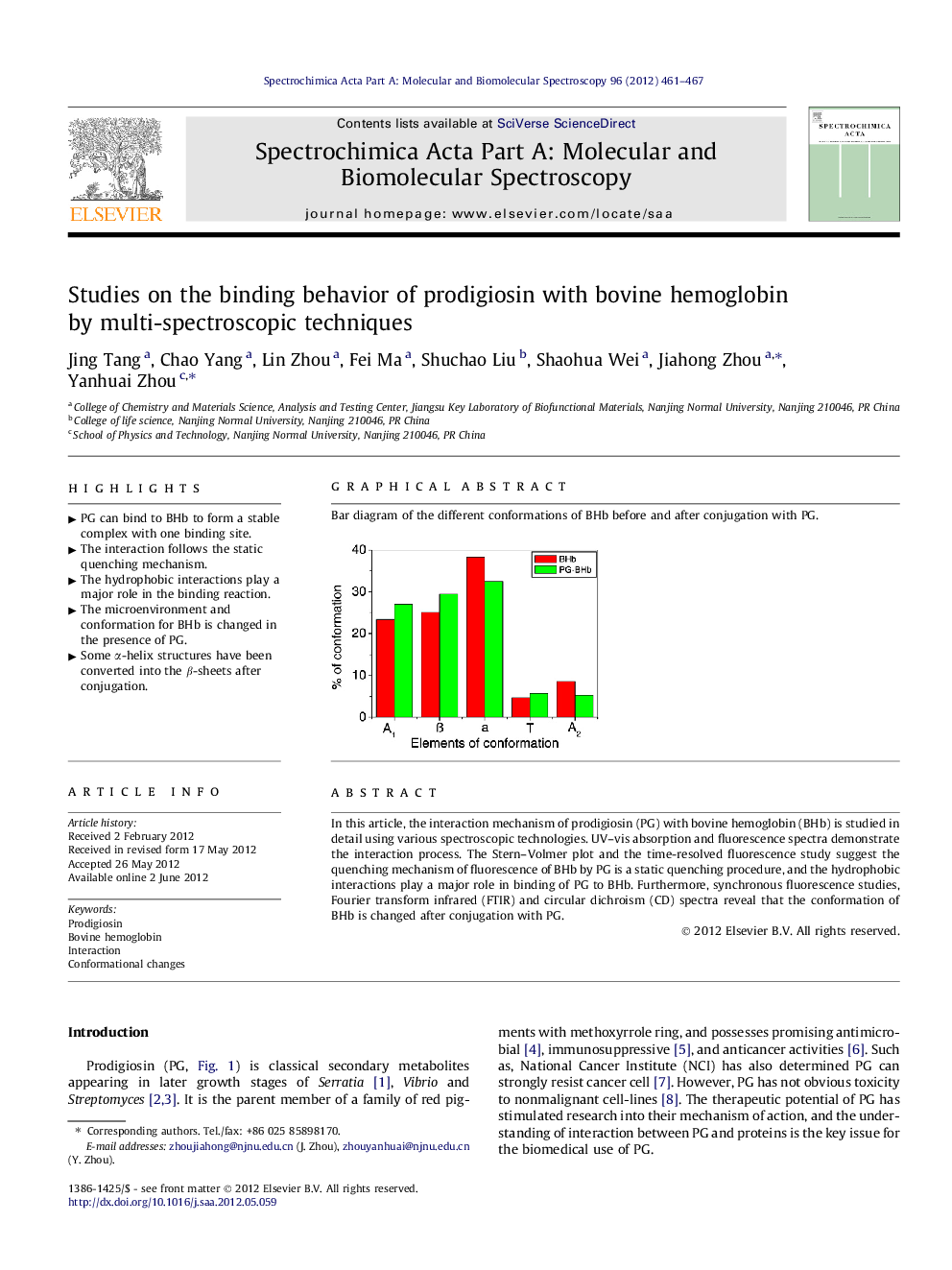
In this article, the interaction mechanism of prodigiosin (PG) with bovine hemoglobin (BHb) is studied in detail using various spectroscopic technologies. UV–vis absorption and fluorescence spectra demonstrate the interaction process. The Stern–Volmer plot and the time-resolved fluorescence study suggest the quenching mechanism of fluorescence of BHb by PG is a static quenching procedure, and the hydrophobic interactions play a major role in binding of PG to BHb. Furthermore, synchronous fluorescence studies, Fourier transform infrared (FTIR) and circular dichroism (CD) spectra reveal that the conformation of BHb is changed after conjugation with PG.
Graphical abstractBar diagram of the different conformations of BHb before and after conjugation with PG.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► PG can bind to BHb to form a stable complex with one binding site. ► The interaction follows the static quenching mechanism. ► The hydrophobic interactions play a major role in the binding reaction. ► The microenvironment and conformation for BHb is changed in the presence of PG. ► Some α-helix structures have been converted into the β-sheets after conjugation.