| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234455 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2012 | 11 Pages |
The sulfonamide compound, 4-methyl-N-(naphthalene-1-yl)benzene sulfonamide (abbreviated as 4MNBS) was synthesized from the reaction of 4-methyl benzene sulfonyl chloride with 1-naphthyl amine. The 4MNBS was characterized by FTIR, 1H NMR, 13C NMR, single crystal X-ray diffraction (XRD) and thermal analysis. By using the Density Functional Theory (DFT) method with 6-31G(d,p) basis set, the molecular geometry and vibrational frequencies were calculated and compared with the experimental data. The title compound crystallizes in the triclinic system of space group p−1 with a = 10.3873(7) Å, b = 10.4090(7) Å, c = 15.7084(10) Å; α = 75.735(3)°; ß = 70.737(3)°; γ = 68.120(3)° and z = 4. Stability of the molecule arising from hyper conjugative interactions, charge delocalization has been analyzed using natural bond orbital (NBO) analysis. In addition, atomic charges, frontier molecular orbitals and molecular electrostatic potential were computed by using Density Functional Theory (DFT/B3LYP) 6-31G(d,p) basis set. The calculated HOMO and LUMO energies show that charge transfer occurs in the molecule.
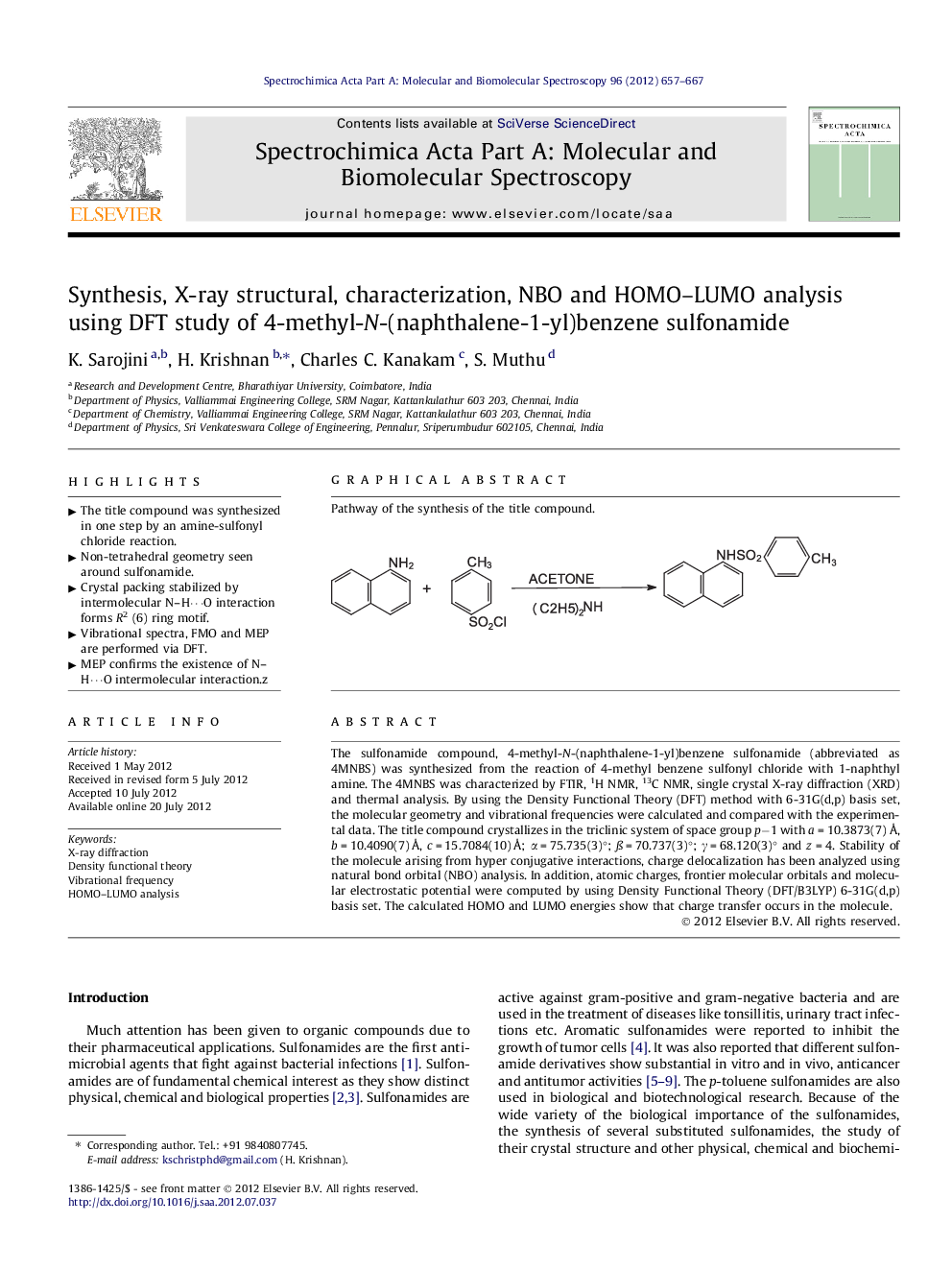
Graphical abstractPathway of the synthesis of the title compound.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The title compound was synthesized in one step by an amine-sulfonyl chloride reaction. ►Non-tetrahedral geometry seen around sulfonamide. ►Crystal packing stabilized by intermolecular N–H⋯O interaction forms R2 (6) ring motif. ► Vibrational spectra, FMO and MEP are performed via DFT. ►MEP confirms the existence of N–H⋯O intermolecular interaction.