| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234751 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 12 Pages |
This work presents the characterization of (2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)piperidine-3,4,5-triol (abbreviated as HEHMPT) by quantum chemical calculations and spectral techniques. The spectroscopic properties were investigated by FT-IR, FT-Raman and UV–Vis techniques. The FT-IR spectrum (4000–400 cm−1) and FT-Raman spectrum (4000–100 cm−1) in solid phase was recorded for HEHMPT. The UV–Vis absorption spectrum of the HEHMPT that dissolved in water was recorded in the range of 100–400 nm. The structural and spectroscopic data of the molecule were obtained from B3LYP and M06-2X with 6-31G(d,p) basis set calculations. The theoretical wavenumbers were scaled and compared with experimental FT-IR and FT-Raman spectra. The complete assignments were performed on the basis of the normal co-ordinate analysis (NCA), experimental results and potential energy distribution (PED) of the vibrational modes, calculated with scaled quantum mechanics (SQM) method, interpreted in terms of fundamental modes. The stable geometry of the compound has been determined from the potential energy surface scan. The stability of molecule has been analyzed by NBO analysis. The molecule orbital contributions were studied by using the total (TDOS), partial (PDOS), and overlap population (OPDOS) density of states. The electronic properties like UV spectral analysis and HOMO–LUMO energies were reported. The calculated HOMO and LUMO energies shows that charge transfer interactions taking place within the molecule. Mulliken population analysis on atomic charges is also calculated.
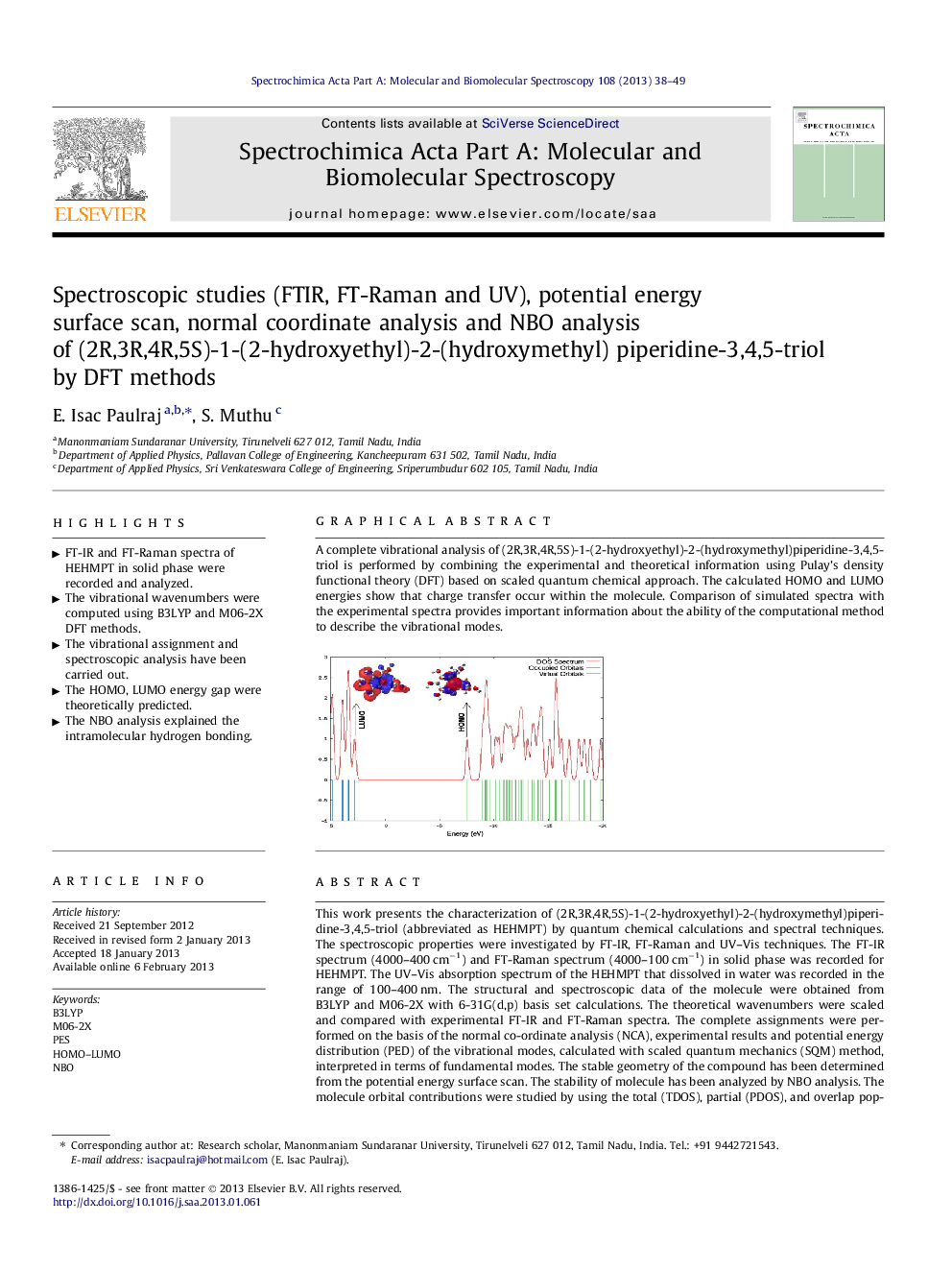
Graphical abstractA complete vibrational analysis of (2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)piperidine-3,4,5-triol is performed by combining the experimental and theoretical information using Pulay’s density functional theory (DFT) based on scaled quantum chemical approach. The calculated HOMO and LUMO energies show that charge transfer occur within the molecule. Comparison of simulated spectra with the experimental spectra provides important information about the ability of the computational method to describe the vibrational modes.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► FT-IR and FT-Raman spectra of HEHMPT in solid phase were recorded and analyzed. ► The vibrational wavenumbers were computed using B3LYP and M06-2X DFT methods. ► The vibrational assignment and spectroscopic analysis have been carried out. ► The HOMO, LUMO energy gap were theoretically predicted. ► The NBO analysis explained the intramolecular hydrogen bonding.