| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1234776 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 5 Pages |
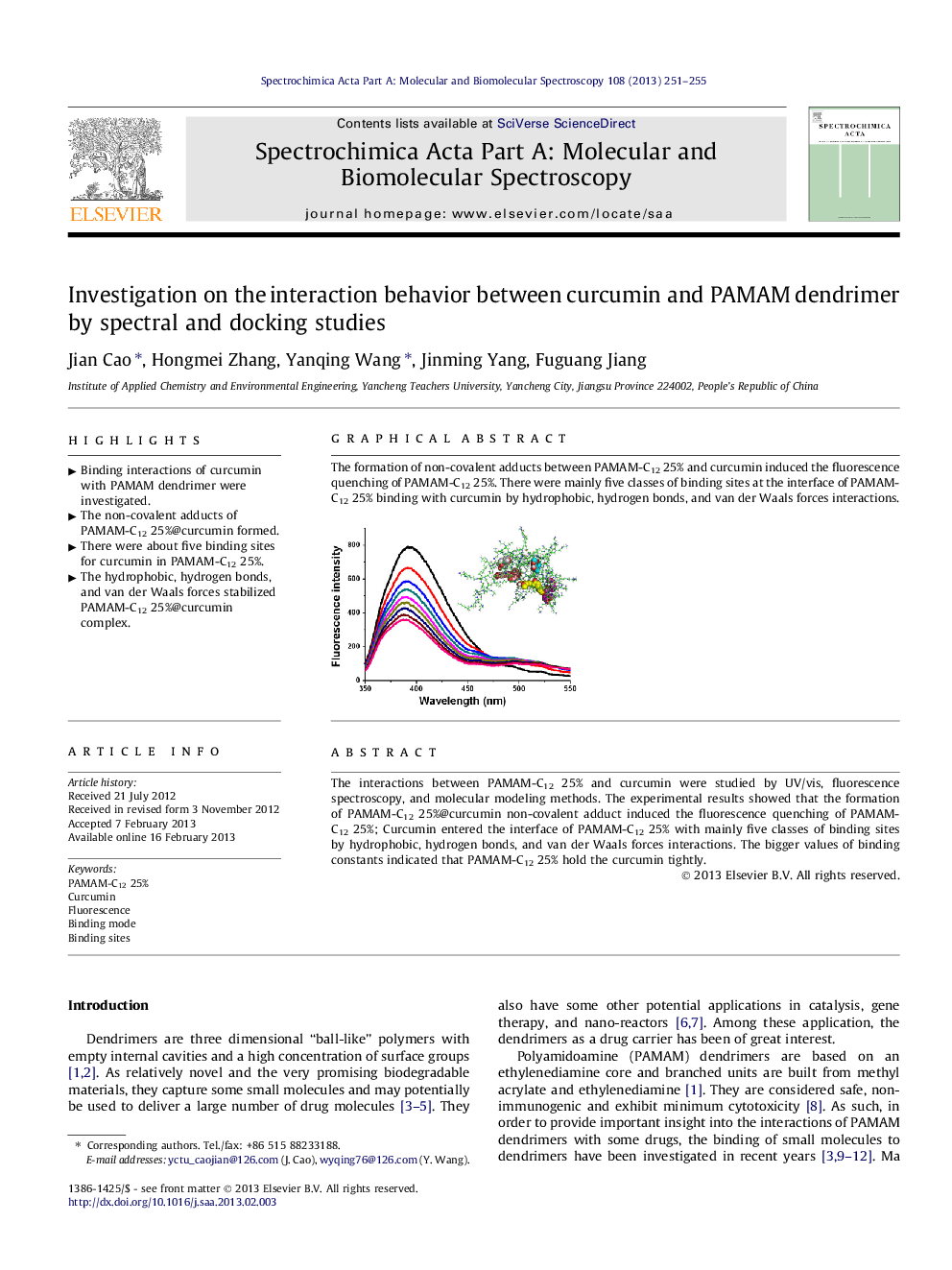
The interactions between PAMAM-C12 25% and curcumin were studied by UV/vis, fluorescence spectroscopy, and molecular modeling methods. The experimental results showed that the formation of PAMAM-C12 25%@curcumin non-covalent adduct induced the fluorescence quenching of PAMAM-C12 25%; Curcumin entered the interface of PAMAM-C12 25% with mainly five classes of binding sites by hydrophobic, hydrogen bonds, and van der Waals forces interactions. The bigger values of binding constants indicated that PAMAM-C12 25% hold the curcumin tightly.
Graphical abstractThe formation of non-covalent adducts between PAMAM-C12 25% and curcumin induced the fluorescence quenching of PAMAM-C12 25%. There were mainly five classes of binding sites at the interface of PAMAM-C12 25% binding with curcumin by hydrophobic, hydrogen bonds, and van der Waals forces interactions.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Binding interactions of curcumin with PAMAM dendrimer were investigated. ► The non-covalent adducts of PAMAM-C12 25%@curcumin formed. ► There were about five binding sites for curcumin in PAMAM-C12 25%. ► The hydrophobic, hydrogen bonds, and van der Waals forces stabilized PAMAM-C12 25%@curcumin complex.