| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1235061 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 9 Pages |
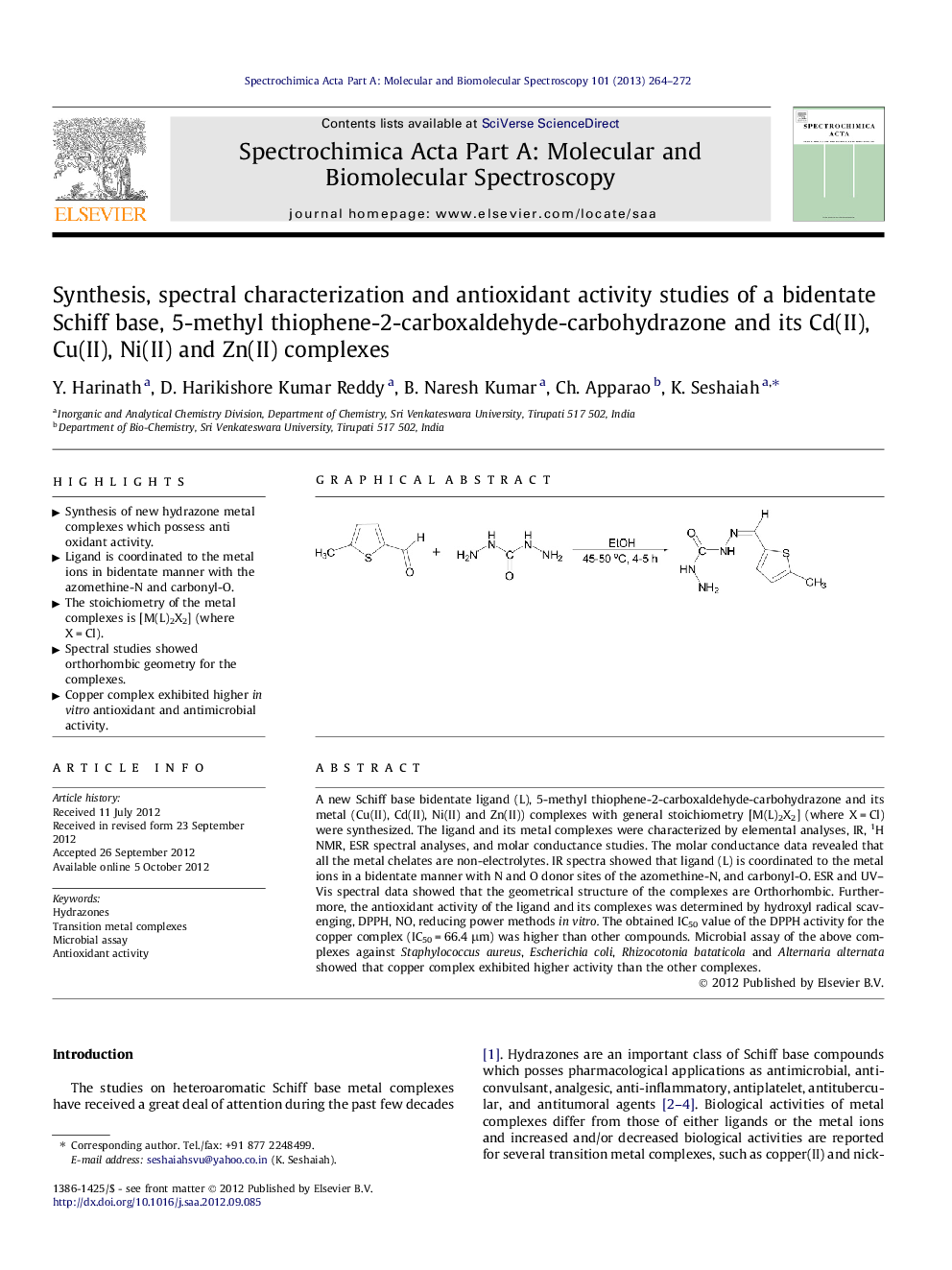
A new Schiff base bidentate ligand (L), 5-methyl thiophene-2-carboxaldehyde-carbohydrazone and its metal (Cu(II), Cd(II), Ni(II) and Zn(II)) complexes with general stoichiometry [M(L)2X2] (where X = Cl) were synthesized. The ligand and its metal complexes were characterized by elemental analyses, IR, 1H NMR, ESR spectral analyses, and molar conductance studies. The molar conductance data revealed that all the metal chelates are non-electrolytes. IR spectra showed that ligand (L) is coordinated to the metal ions in a bidentate manner with N and O donor sites of the azomethine-N, and carbonyl-O. ESR and UV–Vis spectral data showed that the geometrical structure of the complexes are Orthorhombic. Furthermore, the antioxidant activity of the ligand and its complexes was determined by hydroxyl radical scavenging, DPPH, NO, reducing power methods in vitro. The obtained IC50 value of the DPPH activity for the copper complex (IC50 = 66.4 μm) was higher than other compounds. Microbial assay of the above complexes against Staphylococcus aureus, Escherichia coli, Rhizocotonia bataticola and Alternaria alternata showed that copper complex exhibited higher activity than the other complexes.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Synthesis of new hydrazone metal complexes which possess anti oxidant activity. ► Ligand is coordinated to the metal ions in bidentate manner with the azomethine-N and carbonyl-O. ► The stoichiometry of the metal complexes is [M(L)2X2] (where X = Cl). ► Spectral studies showed orthorhombic geometry for the complexes. ► Copper complex exhibited higher in vitro antioxidant and antimicrobial activity.