| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1235069 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
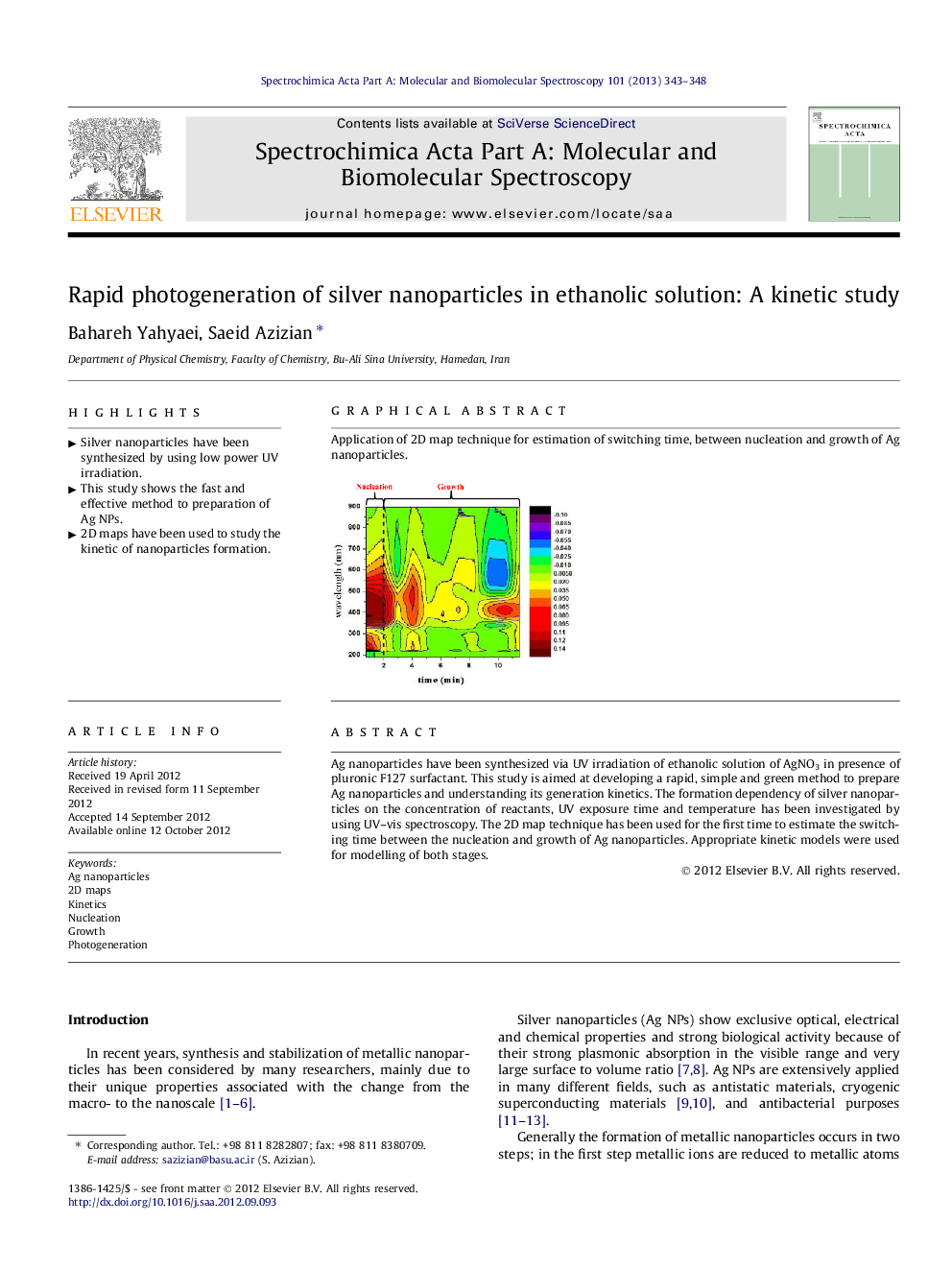
Ag nanoparticles have been synthesized via UV irradiation of ethanolic solution of AgNO3 in presence of pluronic F127 surfactant. This study is aimed at developing a rapid, simple and green method to prepare Ag nanoparticles and understanding its generation kinetics. The formation dependency of silver nanoparticles on the concentration of reactants, UV exposure time and temperature has been investigated by using UV–vis spectroscopy. The 2D map technique has been used for the first time to estimate the switching time between the nucleation and growth of Ag nanoparticles. Appropriate kinetic models were used for modelling of both stages.
Graphical abstractApplication of 2D map technique for estimation of switching time, between nucleation and growth of Ag nanoparticles.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Silver nanoparticles have been synthesized by using low power UV irradiation. ► This study shows the fast and effective method to preparation of Ag NPs. ► 2D maps have been used to study the kinetic of nanoparticles formation.