| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1314946 | Journal of Fluorine Chemistry | 2009 | 4 Pages |
Abstract
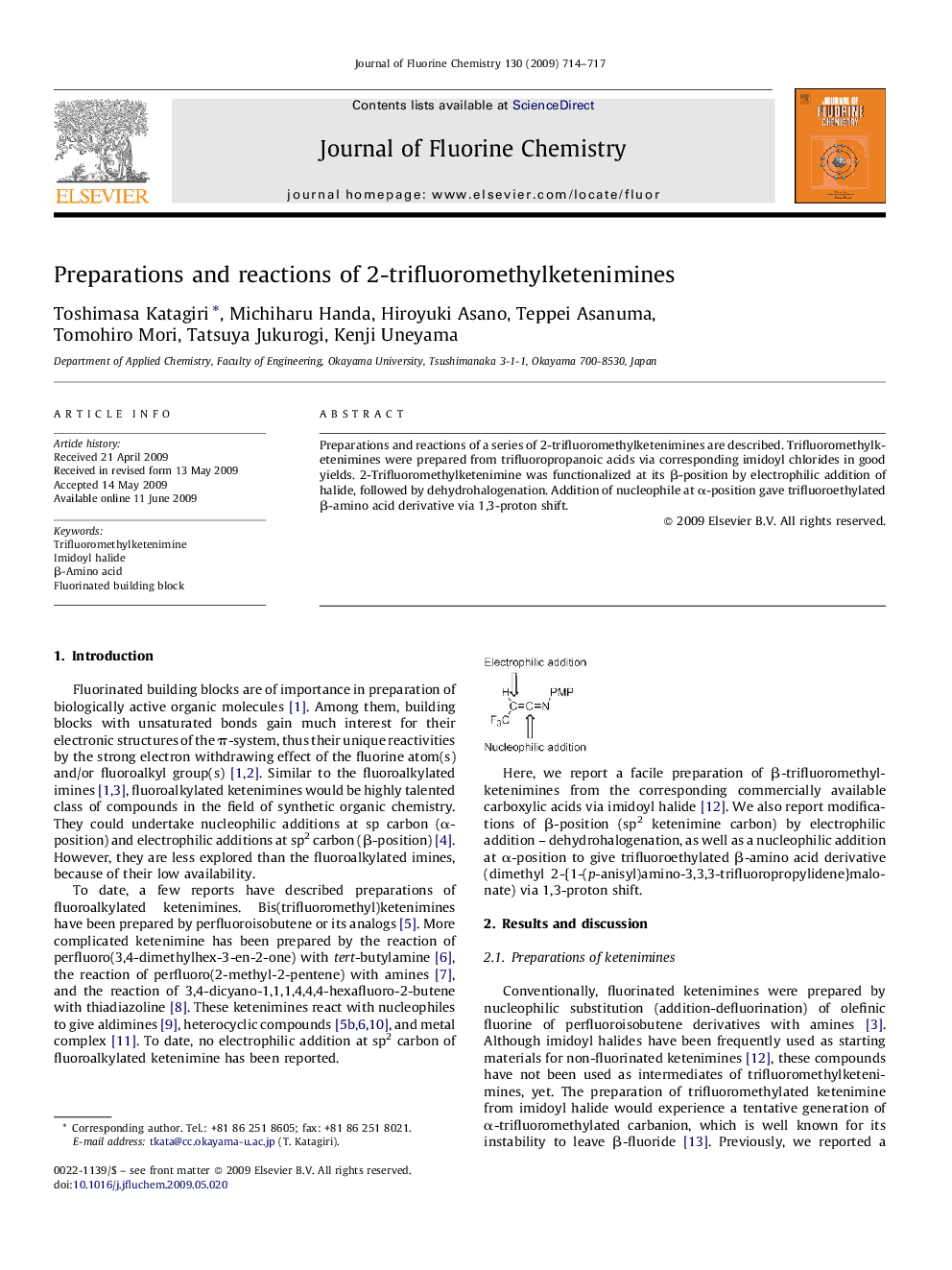
Preparations and reactions of a series of 2-trifluoromethylketenimines are described. Trifluoromethylketenimines were prepared from trifluoropropanoic acids via corresponding imidoyl chlorides in good yields. 2-Trifluoromethylketenimine was functionalized at its β-position by electrophilic addition of halide, followed by dehydrohalogenation. Addition of nucleophile at α-position gave trifluoroethylated β-amino acid derivative via 1,3-proton shift.
Graphical abstract2-Trifluoromethylketenimines are prepared from corresponding acid via imidoyl halides.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Inorganic Chemistry
Authors
Toshimasa Katagiri, Michiharu Handa, Hiroyuki Asano, Teppei Asanuma, Tomohiro Mori, Tatsuya Jukurogi, Kenji Uneyama,