| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1324425 | Journal of Organometallic Chemistry | 2011 | 6 Pages |
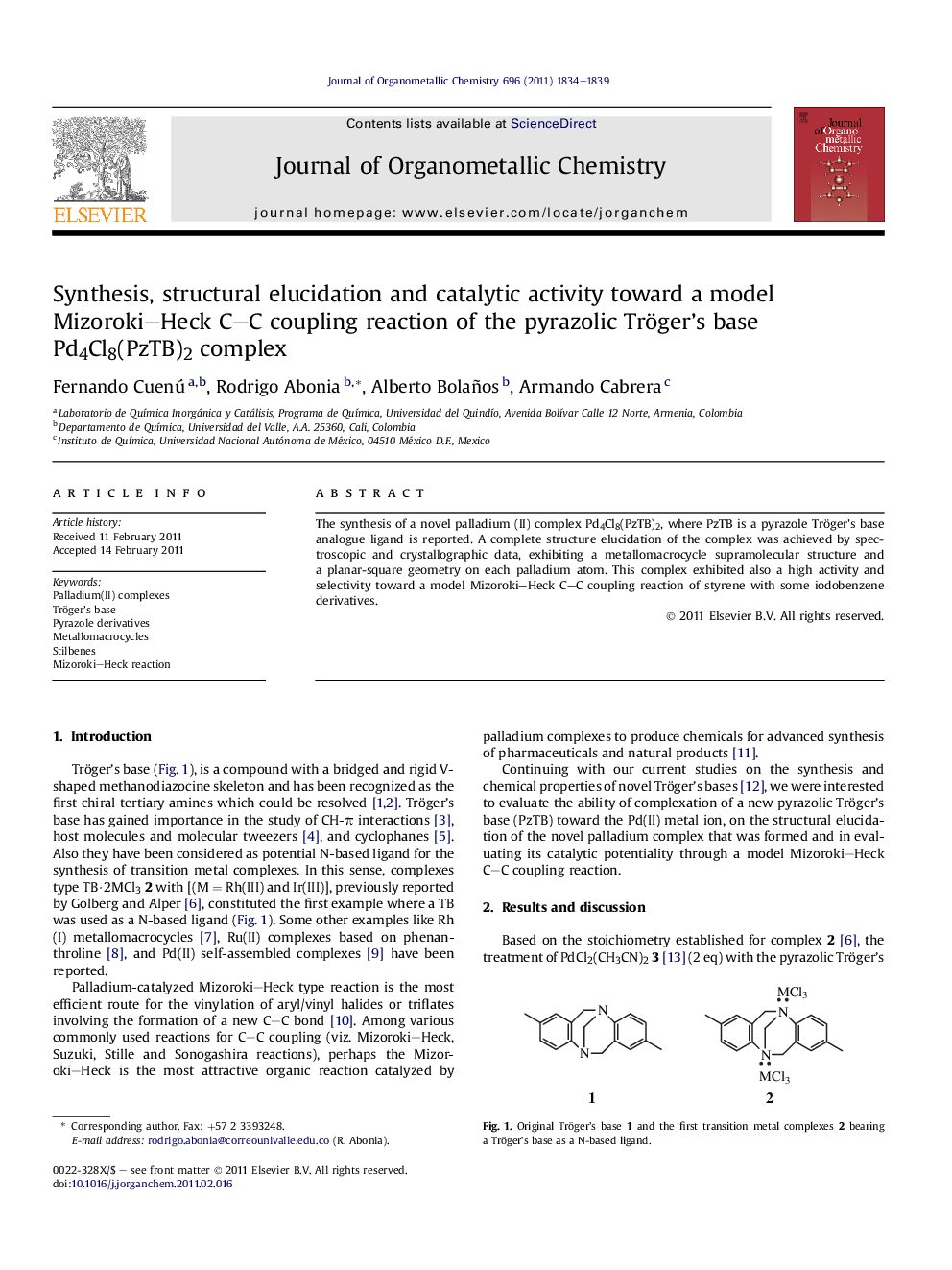
The synthesis of a novel palladium (II) complex Pd4Cl8(PzTB)2, where PzTB is a pyrazole Tröger’s base analogue ligand is reported. A complete structure elucidation of the complex was achieved by spectroscopic and crystallographic data, exhibiting a metallomacrocycle supramolecular structure and a planar-square geometry on each palladium atom. This complex exhibited also a high activity and selectivity toward a model Mizoroki–Heck C–C coupling reaction of styrene with some iodobenzene derivatives.
Graphical abstractThe synthesis of a new metallomacrocycle from the reaction of a pyrazolic Tröger’s base (PzTB) analogue and PdCl2(CH3CN)2 in MeOH at ambient temperature is reported. The structure of this product was unambiguously confirmed by X-ray diffraction and several experiments showed the high catalytic activity of this complex toward the Mizoroki–Heck C–C coupling reaction of styrene with iodobenzene derivatives.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The synthesis of a new metallomacrocycle from the reaction of a pyrazolic Tröger’s base (PzTB) analogue with PdCl2(CH3CN)2 was achieved. ► The structure of this product, Pd4Cl8(PzTB)2, was unambiguously confirmed by X-ray diffraction. ► Several experiments showed the high catalytic activity of this complex toward the Mizoroki–Heck C–C coupling reaction of styrene with iodobenzene derivatives. ► The Hg drop test indicated that this complex behaved as a homogeneous catalyst in the course of the Mizoroki–Heck reactions. ► The increasing of the temperature from 90 °C, 120 °C–160 °C improved the reactivity of this complex showing TOF’s of 12.1, 45.0 and 490.0 respectively.