| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1328216 | Journal of Organometallic Chemistry | 2007 | 12 Pages |
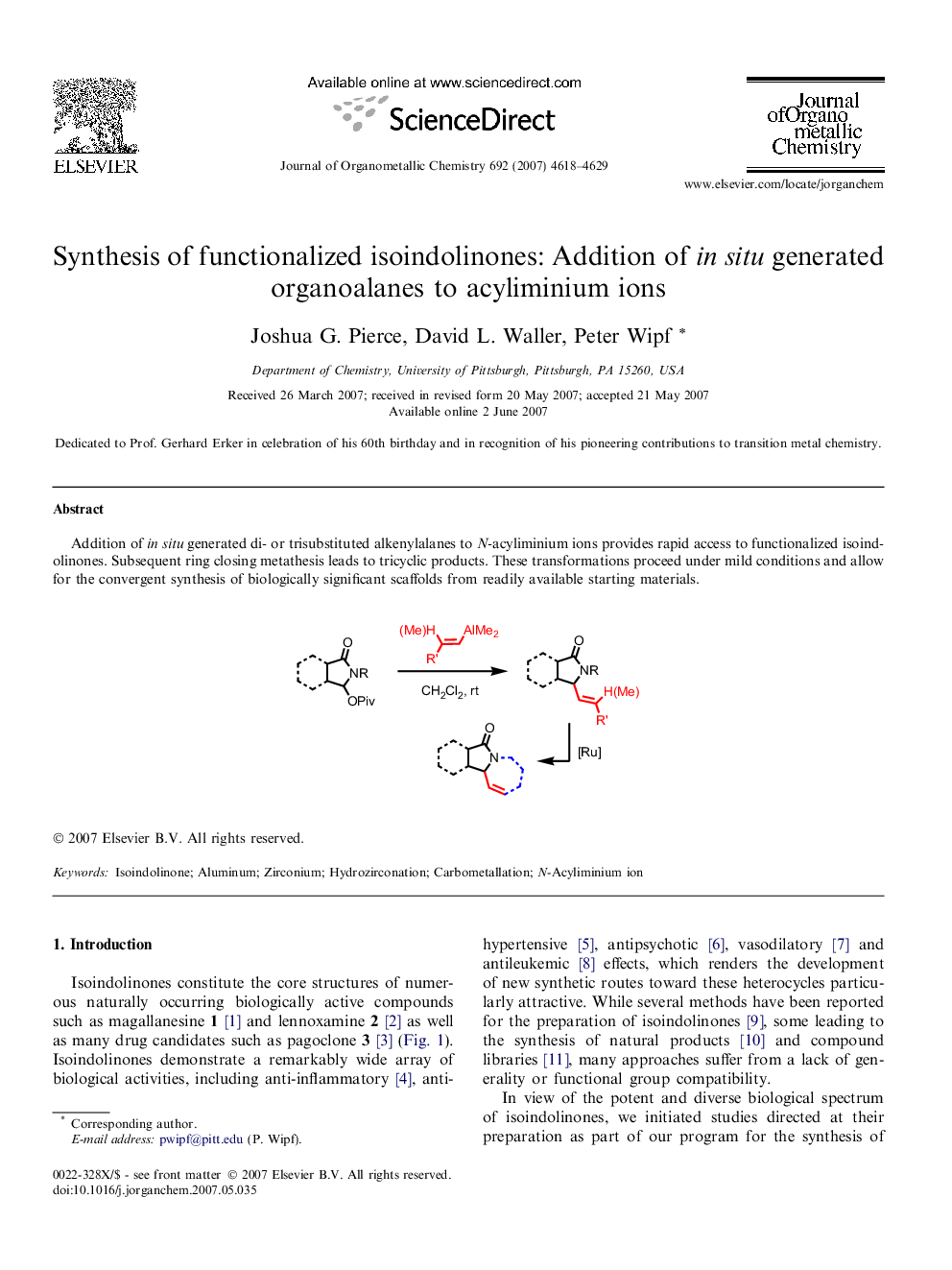
Addition of in situ generated di- or trisubstituted alkenylalanes to N-acyliminium ions provides rapid access to functionalized isoindolinones. Subsequent ring closing metathesis leads to tricyclic products. These transformations proceed under mild conditions and allow for the convergent synthesis of biologically significant scaffolds from readily available starting materials.Figure optionsDownload full-size imageDownload as PowerPoint slide
Graphical abstractAddition of in situ generated di- or trisubstituted alkenylalanes to N-acyliminium ions provides rapid access to functionalized isoindolinones. Subsequent ring closing metathesis leads to tricyclic products. These transformations proceed under mild conditions and allow for the convergent synthesis of biologically significant scaffolds from readily available starting materials.Figure optionsDownload full-size imageDownload as PowerPoint slide