| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1330544 | Journal of Solid State Chemistry | 2011 | 5 Pages |
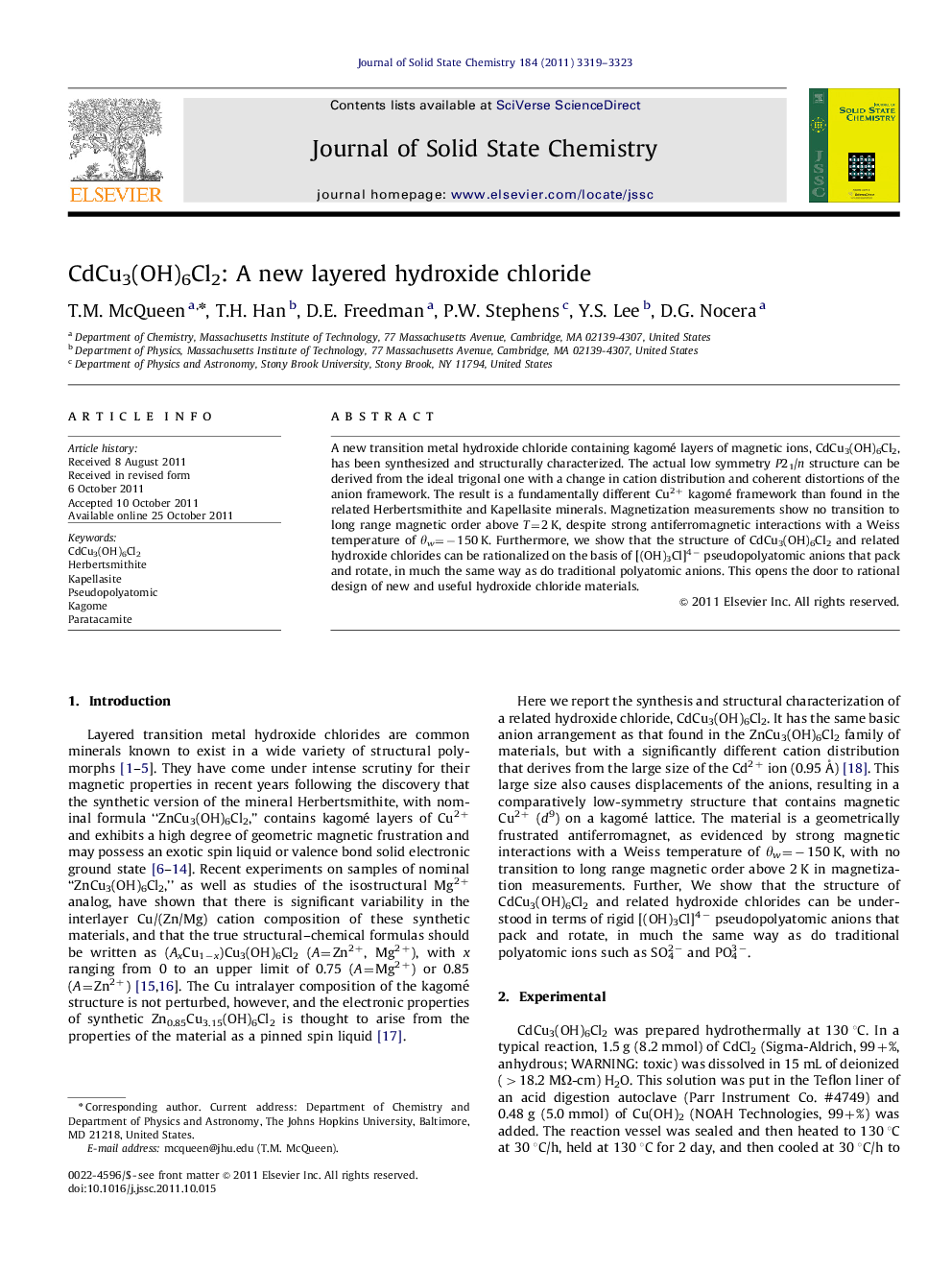
A new transition metal hydroxide chloride containing kagomé layers of magnetic ions, CdCu3(OH)6Cl2, has been synthesized and structurally characterized. The actual low symmetry P21/n structure can be derived from the ideal trigonal one with a change in cation distribution and coherent distortions of the anion framework. The result is a fundamentally different Cu2+ kagomé framework than found in the related Herbertsmithite and Kapellasite minerals. Magnetization measurements show no transition to long range magnetic order above T=2 K, despite strong antiferromagnetic interactions with a Weiss temperature of θw=−150 K. Furthermore, we show that the structure of CdCu3(OH)6Cl2 and related hydroxide chlorides can be rationalized on the basis of [(OH)3Cl]4− pseudopolyatomic anions that pack and rotate, in much the same way as do traditional polyatomic anions. This opens the door to rational design of new and useful hydroxide chloride materials.
Graphical AbstractThe [(OH)3Cl]4− pseudopolyatomic anion and the kagomé lattice of CdCu3[(OH)3Cl]2.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► A new understanding of hydroxide chlorides, based on the polyatomic anion [(OH)3Cl]4−. ► Synthesis and structure of a new layered hydroxide chloride, CdCu3(OH)6Cl2, are reported. ► A new compound is reported with kagomé layers of Cu2+.