| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1336385 | Nano-Structures & Nano-Objects | 2015 | 9 Pages |
•Co-precipitation method produced nanodimensional CexFe1−xO2/CexZr1−xO2 (x:0x:0, 0.25, 0.5, 0.75, 1) oxides.•Phase and morphology transformation of ceria structure depends on dopant concentration.•Synthesized oxides possess nanopores and specific surface area.•Strain dependency with the incorporation of Fe/Zr ions in the ceria lattice.
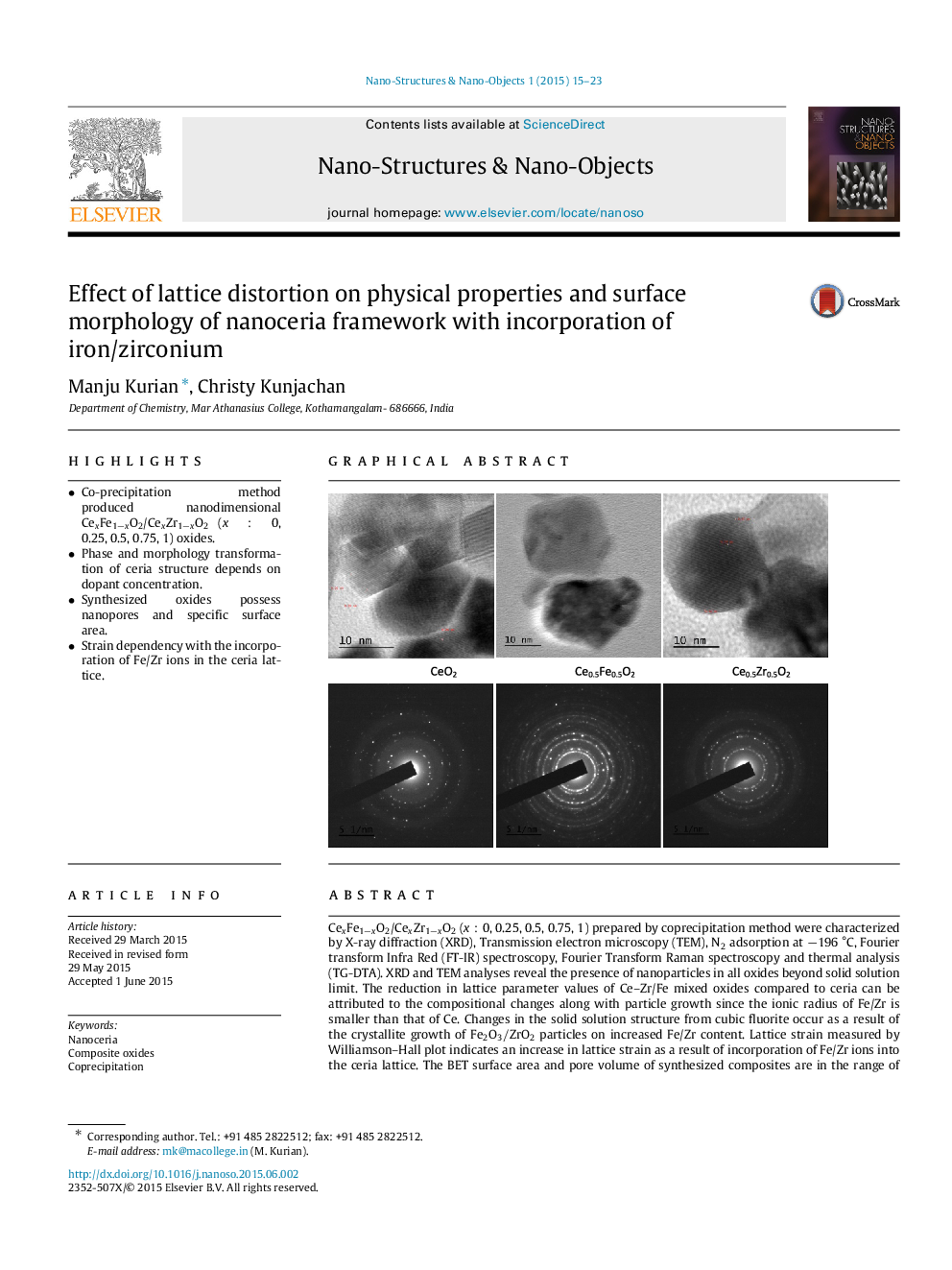
CexFe1−xO2/CexZr1−xO2 (x:0x:0, 0.25, 0.5, 0.75, 1) prepared by coprecipitation method were characterized by X-ray diffraction (XRD), Transmission electron microscopy (TEM), N2 adsorption at −196 °C, Fourier transform Infra Red (FT-IR) spectroscopy, Fourier Transform Raman spectroscopy and thermal analysis (TG-DTA). XRD and TEM analyses reveal the presence of nanoparticles in all oxides beyond solid solution limit. The reduction in lattice parameter values of Ce–Zr/Fe mixed oxides compared to ceria can be attributed to the compositional changes along with particle growth since the ionic radius of Fe/Zr is smaller than that of Ce. Changes in the solid solution structure from cubic fluorite occur as a result of the crystallite growth of Fe2O3/ZrO2 particles on increased Fe/Zr content. Lattice strain measured by Williamson–Hall plot indicates an increase in lattice strain as a result of incorporation of Fe/Zr ions into the ceria lattice. The BET surface area and pore volume of synthesized composites are in the range of 30–10m2/g and 0.004–0.01cm3/g. Raman analysis reveals that iron incorporated ceria lattice have increased oxygen vacancy formation and gradual shrinkage of the unit cell. FT-IR spectra reveal the characteristic Ce–O stretching and crystalline water absorption of synthesized nanoparticles. Thermal analysis data shows these oxides to be thermally stable.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide