| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1343631 | Tetrahedron: Asymmetry | 2015 | 5 Pages |
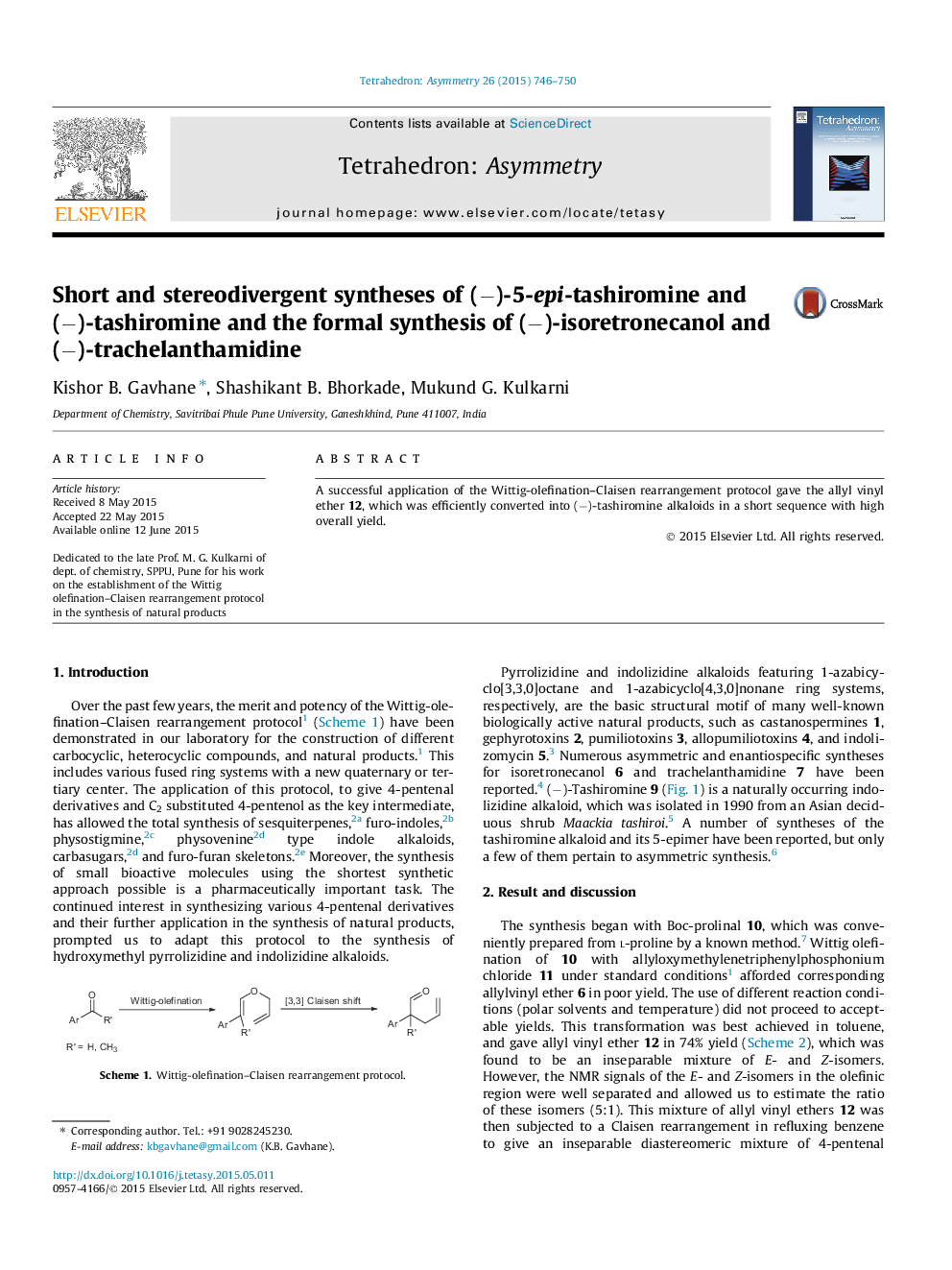
A successful application of the Wittig-olefination–Claisen rearrangement protocol gave the allyl vinyl ether 12, which was efficiently converted into (−)-tashiromine alkaloids in a short sequence with high overall yield.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
tert-Butyl (S)-2-((S)-1-hydroxypent-4-en-2-yl)pyrrolidine-1-carboxylateC14H25NO3[α]D25 = −19.3 (c 1.0, CHCl3)Source of chirality: (S)-prolineAbsolute configuration (S,S)
tert-Butyl (S)-2-((R)-1-hydroxypent-4-en-2-yl)pyrrolidine-1-carboxylateC14H25NO3[α]D25 = −26.7 (c 1.02, CHCl3)Source of chirality: (S)-prolineAbsolute configuration (S,R)
tert-Butyl (S)-2-((S)-1-(benzyloxy)pent-4-en-2-yl)pyrrolidine-1-carboxylateC21H31NO3[α]D25 = −16.9 (c 1.4, CHCl3)Source of chirality: (S)-prolineAbsolute configuration (S,S)
tert-Butyl (S)-2-((R)-1-(benzyloxy)pent-4-en-2-yl)pyrrolidine-1-carboxylateC21H31NO3[α]D25 = −23.2 (c 0.8, CHCl3)Source of chirality: (S)-prolineAbsolute configuration (S,R)
tert-Butyl (S)-2-((S)-1-(benzyloxy)-5-hydroxypentan-2-yl)pyrrolidine-1-carboxylateC21H33NO4[α]D25 = −28.1 (c 1.1, CHCl3)Source of chirality: (S)-prolineAbsolute configuration (S,S)
tert-Butyl (S)-2-((R)-1-(benzyloxy)-5-hydroxypentan-2-yl)pyrrolidine-1-carboxylateC21H33NO4[α]D25 = −34.6 (c 0.9, CHCl3)Source of chirality: (S)-prolineAbsolute configuration (S,R)
(8S,8aS)-8-((Benzyloxy)methyl)octahydroindolizineC16H23NO[α]D25 = −9.3 (c 1.6, MeOH)Source of chirality: (S)-prolineAbsolute configuration (S,S)
(8R,8aS)-8-((Benzyloxy)methyl)octahydroindolizineC16H23NO[α]D25 = −48.2 (c 0.8, MeOH)Source of chirality: (S)-prolineAbsolute configuration (S,R)
((8S,8aS)-Octahydroindolizin-8-yl)methanolC9H17NO[α]D25 = −1.9 (c 1.0, EtOH)Source of chirality: (S)-prolineAbsolute configuration (S,S)
((8R,8aS)-Octahydroindolizin-8-yl)methanolC9H17NO[α]D25 = −41.6 (c 0.9 EtOH)Source of chirality: (S)-prolineAbsolute configuration (S,R)