| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344035 | Tetrahedron: Asymmetry | 2012 | 5 Pages |
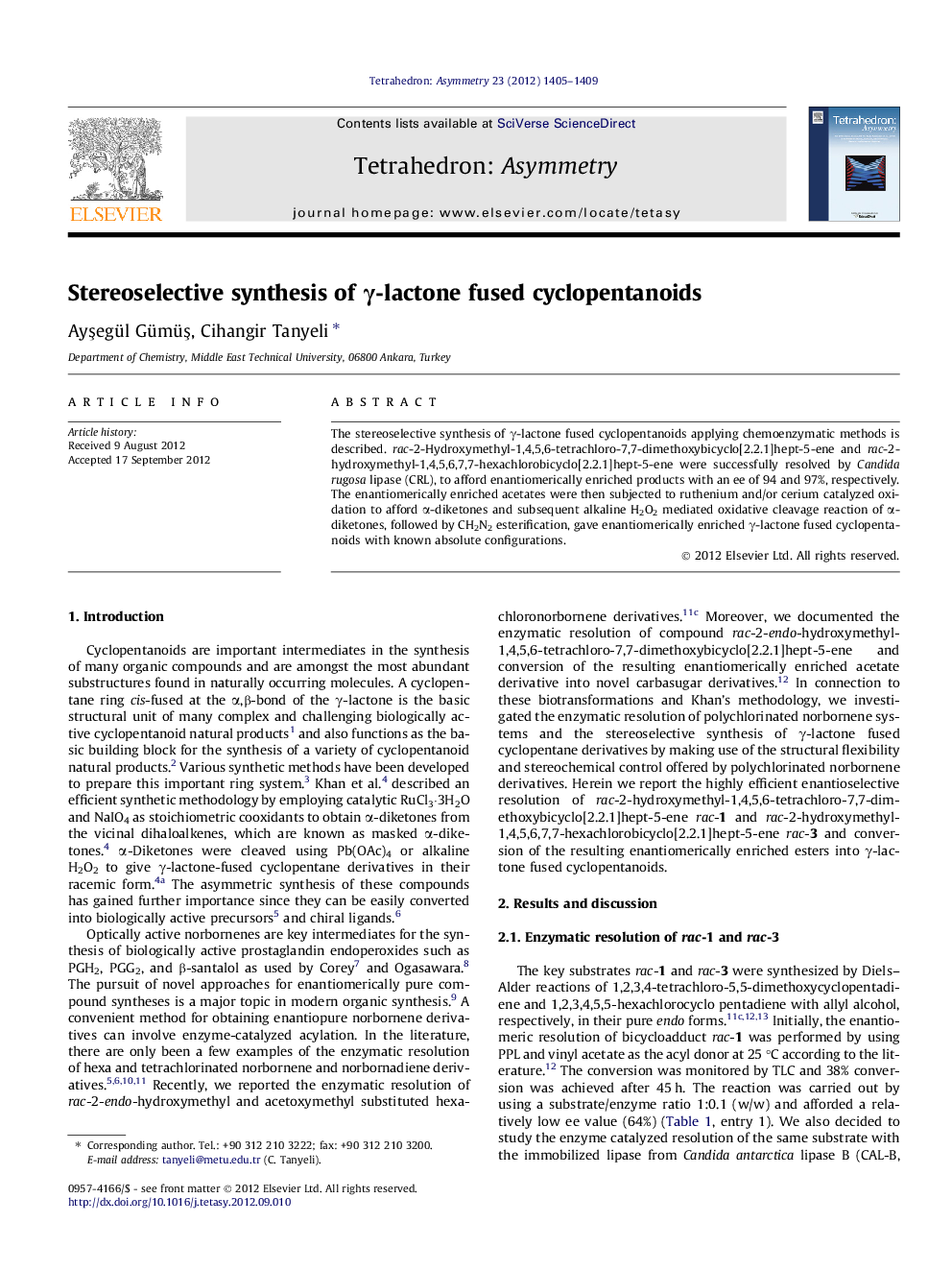
The stereoselective synthesis of γ-lactone fused cyclopentanoids applying chemoenzymatic methods is described. rac-2-Hydroxymethyl-1,4,5,6-tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-ene and rac-2-hydroxymethyl-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene were successfully resolved by Candida rugosa lipase (CRL), to afford enantiomerically enriched products with an ee of 94 and 97%, respectively. The enantiomerically enriched acetates were then subjected to ruthenium and/or cerium catalyzed oxidation to afford α-diketones and subsequent alkaline H2O2 mediated oxidative cleavage reaction of α-diketones, followed by CH2N2 esterification, gave enantiomerically enriched γ-lactone fused cyclopentanoids with known absolute configurations.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
((1S,2R,4R)-1,4-Dichloro-7,7-dimethoxy-5,6-dioxobicyclo[2.2.1]heptan-2-yl)methyl acetateC12H14Cl2O6ee = 94%[α]D20=+24.6 (c 3.7, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (1S,2R,4R)
((1S,2R,4R)-1,4,7,7-Tetrachloro-5,6-dioxobicyclo[2.2.1]heptan-2-yl)methyl acetateC10H8Cl4O4ee = 97%[α]D20=-46.0 (c 1.0, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (1S,2R,4R)
(3aR,5S,6aR)-Methyl 5,6a-dichloro-6,6-dimethoxy-1-oxohexahydro-1H-cyclopenta[c]furan-5-carboxylateC11H14Cl2O6ee = 94%[α]D20=+7.2 (c 0.6, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3aR,5S,6aR)
(3aR,5R,6aS)-Methyl 5,6,6,6a-tetrachloro-1-oxohexahydro-1H-cyclopenta[c]furan-5-carboxylateC9H8Cl4O4ee = 97%[α]D20=-8.3(c 0.6, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3aR,5R,6aS)
(1R,3S,4R)-Dimethy 4-(acetoxymethyl)-1,2,2,3-tetrachloro cyclopentane-1,3-dicarboxylateC12H14Cl4O6ee = 97%[α]D20=-17.4 (c 2.5, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (1R,3S,4R)