| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344128 | Tetrahedron: Asymmetry | 2012 | 6 Pages |
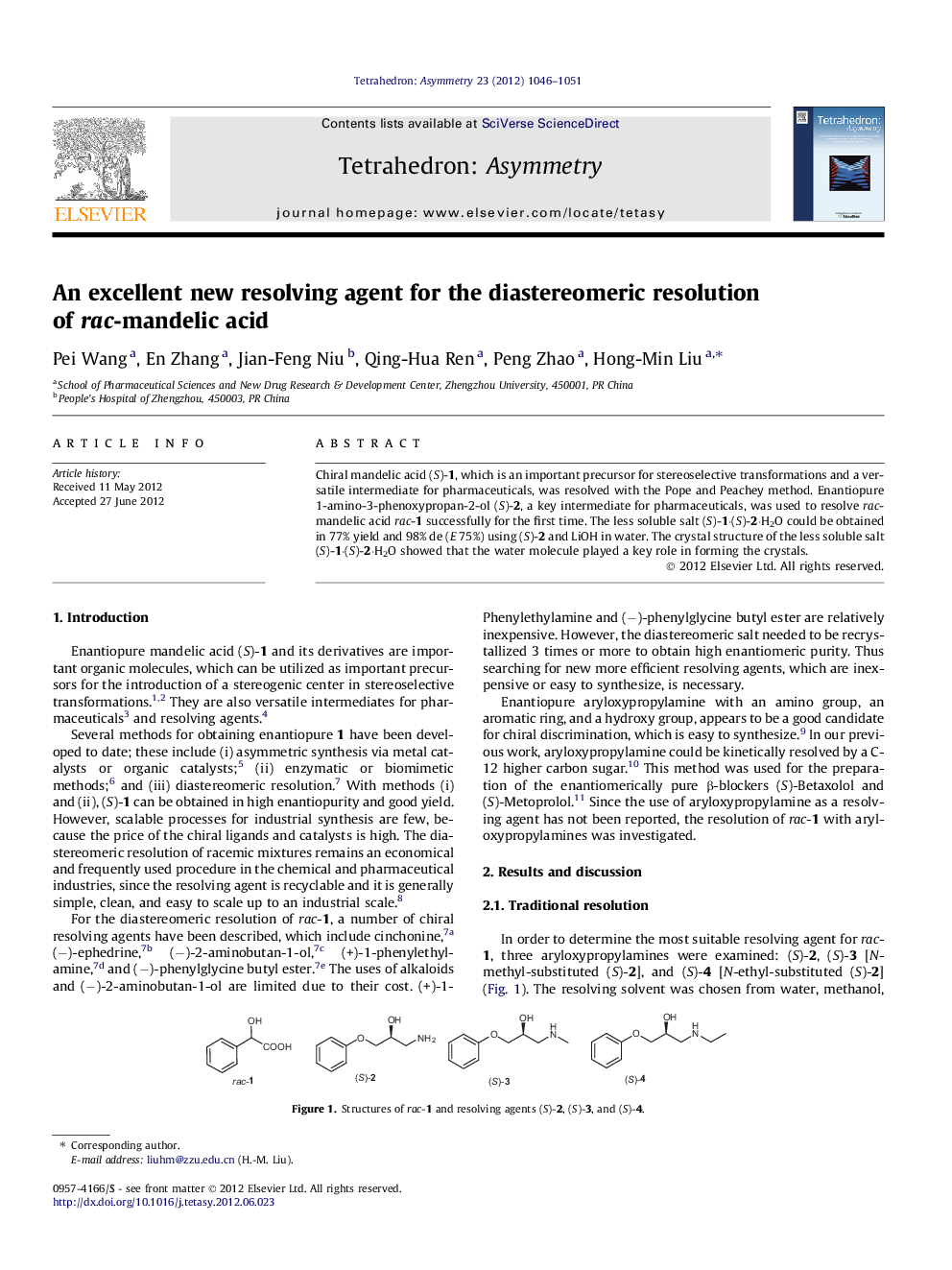
Chiral mandelic acid (S)-1, which is an important precursor for stereoselective transformations and a versatile intermediate for pharmaceuticals, was resolved with the Pope and Peachey method. Enantiopure 1-amino-3-phenoxypropan-2-ol (S)-2, a key intermediate for pharmaceuticals, was used to resolve rac-mandelic acid rac-1 successfully for the first time. The less soluble salt (S)-1·(S)-2·H2O could be obtained in 77% yield and 98% de (E 75%) using (S)-2 and LiOH in water. The crystal structure of the less soluble salt (S)-1·(S)-2·H2O showed that the water molecule played a key role in forming the crystals.
Graphical abstractChiral mandelic acid (S)-1, which is an important precursor for stereoselective transformations and a versatile intermediate for pharmaceuticals, was resolved with the Pope and Peachey method using (S)-2 as resolving agent. The less soluble salt (S)-1·(S)-2·H2O could be obtained in 77% yield and 98% de (E 75%).Figure optionsDownload full-size imageDownload as PowerPoint slide
(S)-Mandelic acid·(S)-1-amino-3-phenoxypropan-2-ol·H2OC17H23NO6Enantiomeric excess of liberated mandelic acid: ee = 98% [by HPLC][α]D23=+27.0 (c 1.0, ethanol)Source of chirality of mandelic acid: resolved by (S)-1-amino-3-phenoxypropan-2-olAbsolute configuration of liberated mandelic acid: (S)
(S)-1-Amino-3-phenoxypropan-2-olC9H13NO2Enantiomeric excess: ee = 100% [by HPLC][α]D24=-10.5 (c 1.0, ethanol)Source of chirality: (S)-epichlorohydrinAbsolute configuration: (S)
(S)-N-Methyl-1-amino-3-phenoxypropan-2-olC10H15NO2Enantiomeric excess: ee = 100% [by HPLC][α]D25=-16.0 (c 1.0, ethanol)Source of chirality: (S)-epichlorohydrinAbsolute configuration: (S)
(S)-N-Ethyl-1-amino-3-phenoxypropan-2-olC11H17NO2Enantiomeric excess: ee = 100% [by HPLC][α]D19=-13.0 (c 1.0, ethanol)Source of chirality: (S)-epichlorohydrinAbsolute configuration: (S)