| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344132 | Tetrahedron: Asymmetry | 2012 | 4 Pages |
Abstract
A novel regio- and diastereospecific ring cleavage of bornane-2,3-dione (camphor quinone) under Bucherer-Bergs reaction conditions has been investigated. The simplicity of this transformation provides a novel and straightforward synthetic pathway to enantiopure derivatives of cyclopentane carboxylic acid as well as functionalized hydantoins in just two steps, starting from inexpensive and easily available camphor.
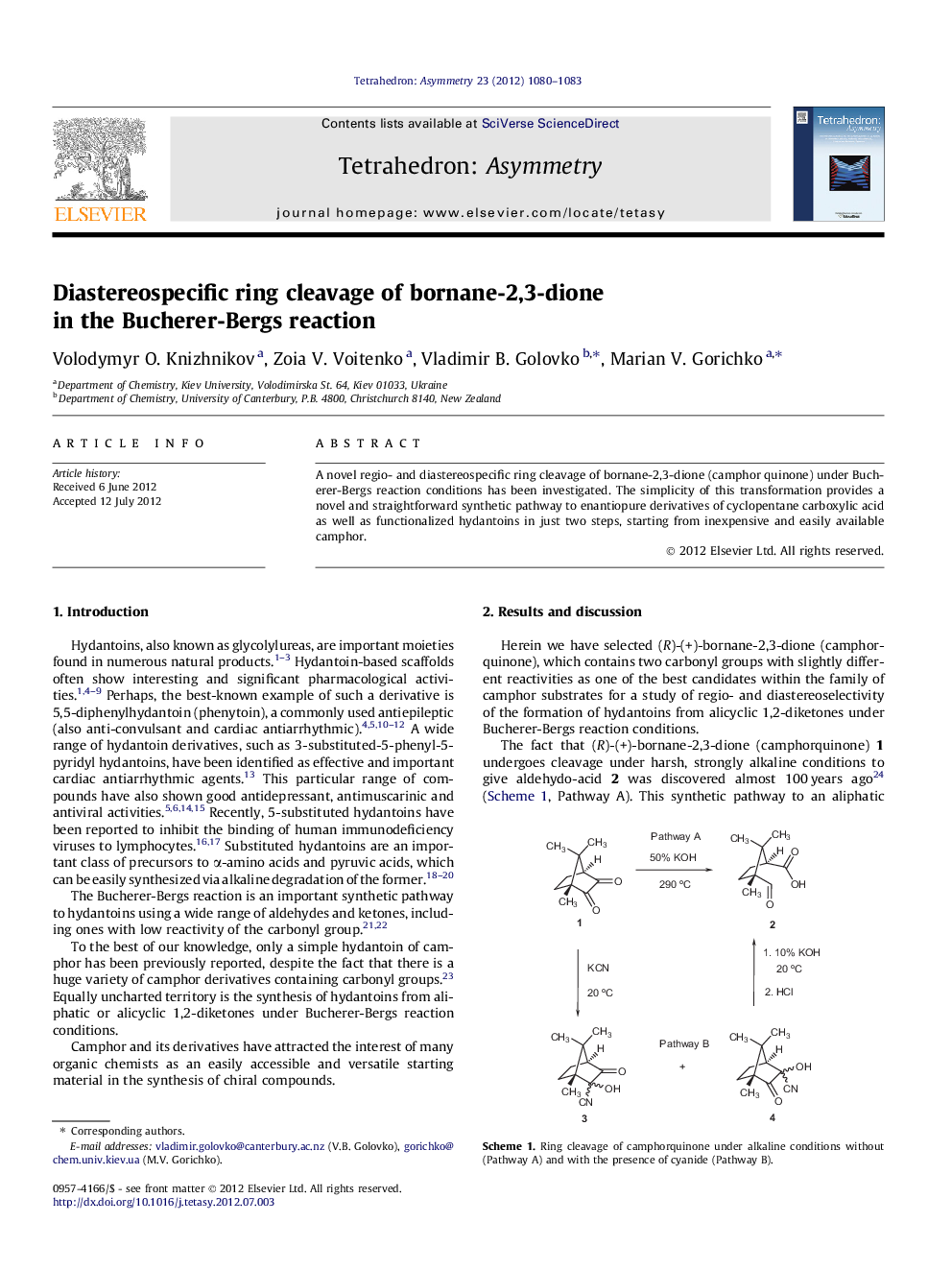
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1R,3S)-3-[(4R)-2,5-Dioxoimidazolidin-4-yl]-1,2,2-trimethylcyclopentanecarboxamideC12H19N3O3de = 100%[α]D28=+156.0 (c 0.50, DMSO)Source of chirality: (R)-(+)-camphorquinoneAbsolute configuration: (1R,3S,4R)
Related Topics
Physical Sciences and Engineering
Chemistry
Inorganic Chemistry
Authors
Volodymyr O. Knizhnikov, Zoia V. Voitenko, Vladimir B. Golovko, Marian V. Gorichko,