| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344134 | Tetrahedron: Asymmetry | 2012 | 7 Pages |
A chemoenzymatic asymmetric synthesis of the title compound has been developed using an efficient lipase-catalyzed acylation and a chiral reagent-directed diastereoselective allylation as the key steps for incorporating the stereogenic centers. A cross metathesis was employed to obtain the required E-olefin geometry of the target compound.
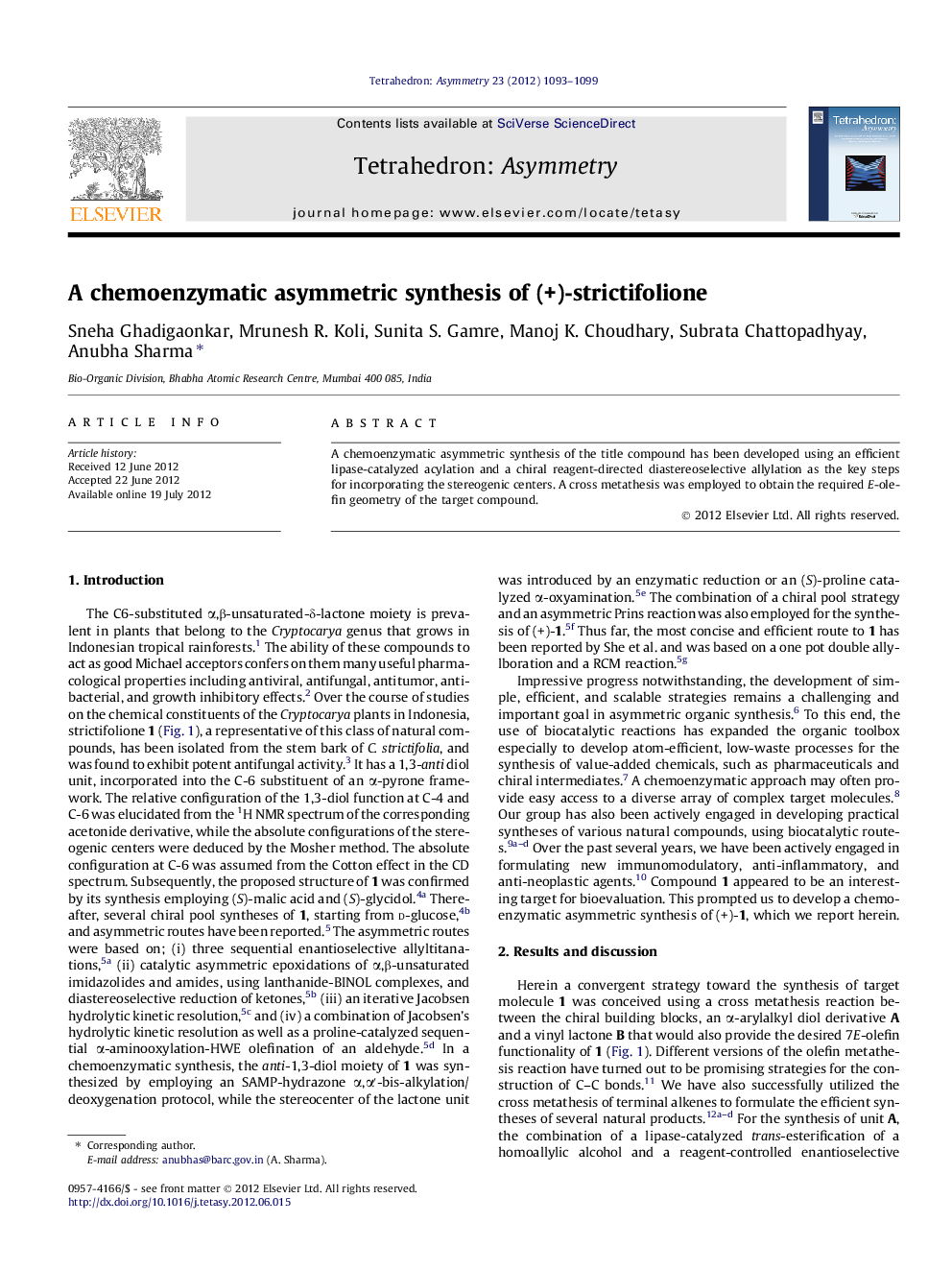
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-3-Acetoxy-1-phenylhex-5-eneC14H18O2[α]D22=-6.6 (c 1.01, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (S)
(S)-1-Phenylhex-5-ene-3-olC12H16O[α]D22=-18.7 (c 1.01, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (S)
(S)-4-tert-Butyldimethylsilyloxy-6-phenylhex-5-eneC18H30OSi[α]D22=-5.5 (c 1.17, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (S)
(S)-3-tert-Butyldimethylsilyloxy-5-phenylpentanalC17H28O2Si[α]D22=+11.1 (c 1.14, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (S)
(4S,6S)-6-tert-Butyldimethylsilyloxy-8-phenyloct-1-en-4-olC20H34O2Si[α]D22=+5.8 (c 1.09, CHCl3)Source of chirality: lipase-catalyzed esterification and asymmetric allylationAbsolute configuration: (4S,6S)
(4S,6S)-8-Phenyloct-1-en-4,6-diolC14H20O2[α]D22=+10.2 (c 1.02, CHCl3)Source of chirality: lipase-catalyzed esterification and asymmetric allylationAbsolute configuration: (4S,6S)
(R)-5-(tert)-Butyldimethylsilyloxypent-1-en-3-olC11H24O2Si[α]D22=-8.3 (c 1.71, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (R)
(3S)-5-(tert)-Butyldimethylsilyloxy -3-acetoxypent-1-eneC13H26O3Si[α]D22=-15.0 (c 1.51, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (3S)
(5R,2Z)-Hepta-2,6-dien-5-olideC7H8O2[α]D22=+92.7 (c 1.14, CHCl3)Source of chirality: lipase-catalyzed esterificationAbsolute configuration: (5R)
(+)-(5R,9S,11S)-StrictifolioneC19H24O4[α]D22=+35.4 (c 1.14, CHCl3)Source of chirality: lipase-catalyzed esterification and asymmetric allylationAbsolute configuration: 5R,9S,11S