| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344268 | Tetrahedron: Asymmetry | 2012 | 6 Pages |
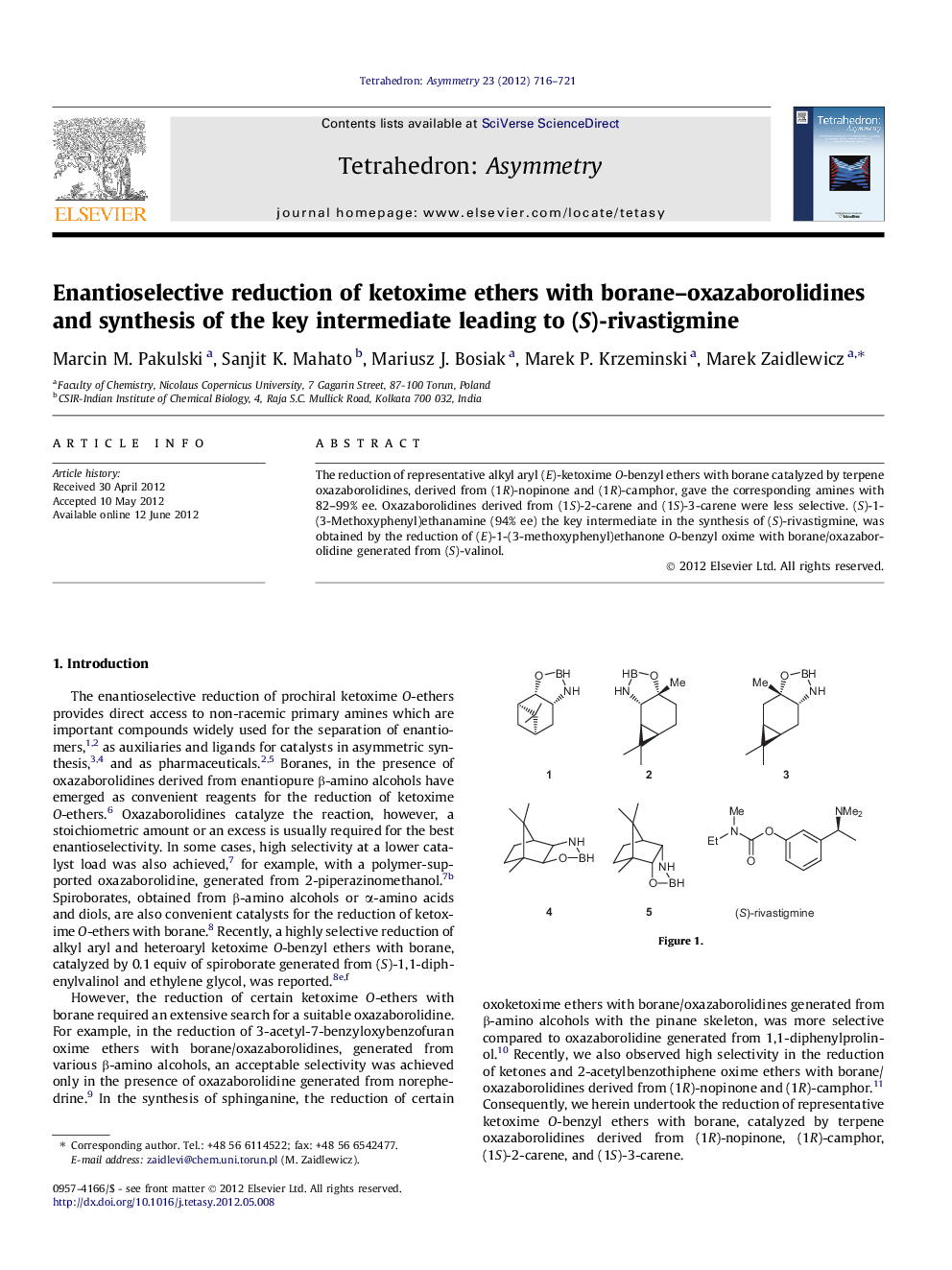
The reduction of representative alkyl aryl (E)-ketoxime O-benzyl ethers with borane catalyzed by terpene oxazaborolidines, derived from (1R)-nopinone and (1R)-camphor, gave the corresponding amines with 82–99% ee. Oxazaborolidines derived from (1S)-2-carene and (1S)-3-carene were less selective. (S)-1-(3-Methoxyphenyl)ethanamine (94% ee) the key intermediate in the synthesis of (S)-rivastigmine, was obtained by the reduction of (E)-1-(3-methoxyphenyl)ethanone O-benzyl oxime with borane/oxazaborolidine generated from (S)-valinol.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-1-(3-Methoxyphenyl)ethanamineC9H13NOEe = 94% from GC[α]D20=-18.8 (c 1.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(R)-1-(Phenyl)ethanamineC8H11NEe = 85% from GC[α]D20=+25.1 (c 0.6, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-1-(4-Methoxyphenyl)ethanamineC9H13NOEe = 99% from GC[α]D20=+28.9 (c 2.0, benzene)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-1(4-Benzyloxyphenyl)ethanamineC15H17NOEe = 81.6% from HPLC[α]D20=+22.8 (c 1.75, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-1-(4-Chlorophenyl)ethanamineC8H10ClNEe = 91% from GC[α]D20=+21.8 (c 2.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-1-(4-Fluorophenyl)ethanamineC8H10FNEe = 84.2% from GC[α]D20=+21.4 (c 1.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-2,3-Dihydro-1H-inden-1-amineC9H11NEe = 69.5% from GC[α]D20=-12.2 (c 1.0, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-1,2,3,4-Tetrahydronaphthalen-1-amineC10H13NEe = 40% from GC[α]D20=-12.1 (c 1.3, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (R)