| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344363 | Tetrahedron: Asymmetry | 2011 | 11 Pages |
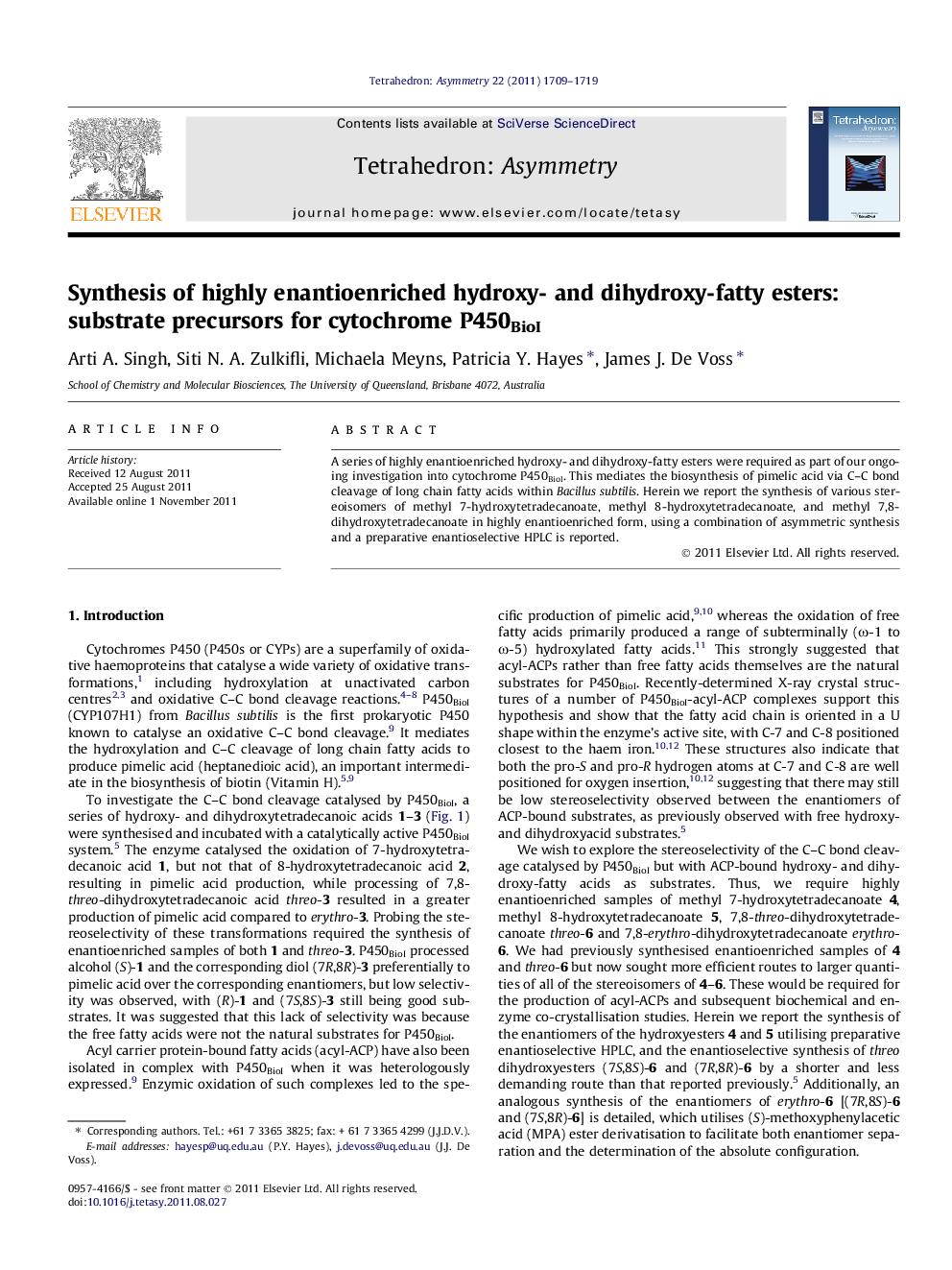
A series of highly enantioenriched hydroxy- and dihydroxy-fatty esters were required as part of our ongoing investigation into cytochrome P450BioI. This mediates the biosynthesis of pimelic acid via C–C bond cleavage of long chain fatty acids within Bacillus subtilis. Herein we report the synthesis of various stereoisomers of methyl 7-hydroxytetradecanoate, methyl 8-hydroxytetradecanoate, and methyl 7,8-dihydroxytetradecanoate in highly enantioenriched form, using a combination of asymmetric synthesis and a preparative enantioselective HPLC is reported.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(R)-14-Methoxy-14-oxotetradec-8-yn-7-yl benzoateC22H30O496% ee[α]D25=+17.3 (c 0.93, CHCl3)Absolute configuration: (8R)Source of chirality: enantioselective HPLC
(R)-Methyl 8-hydroxytetradec-6-ynoateC15H26O3[α]D25=-1.7 (c 0.46, CHCl3)Absolute configuration: (8R)Source of chirality: enantioselective HPLC
(R)-Methyl 8-hydroxytetradecanoateC15H30O3[α]D25=-1.2 (c 0.68, CHCl3)Absolute configuration: (8R)Source of chirality: enantioselective HPLC
(R)-1-Methoxy-1-oxotetradec-5-yn-7-yl benzoateC22H30O4>99% ee[α]D25=+20.4 (c 1.20, CHCl3)Absolute configuration: (7R)Source of chirality: enantioselective HPLC
(R)-Methyl 7-hydroxytetradecanoateC15H30O3[α]D25=-1.6 (c 0.95, CHCl3)Absolute configuration: (7R)Source of chirality: enantioselective HPLC
(7R,8R)-Methyl 7,8-dihydroxytetradecanoateC15H30O4>99% ee[α]D25=+21.0 (c 0.51, MeOH)Absolute configuration: (7R,8R)Source of chirality: Sharpless asymmetric dihydroxylation
(7R,8S)-Methyl 7,8-bis((S)-2-methoxy-2 phenylacetoxy)tetradecanoateC33H46O8>99% ee, >99% de[α]D25=+72.2 (c 0.34, CHCl3)Absolute configuration: (7R,8S,2′S,2″S)Source of chirality: (S)-methoxyphenylacetic acid
(7S,8R)-Methyl 7,8-bis((S)-2-methoxy-2 phenylacetoxy)tetradecanoateC33H46O8Enantiomeric excess: >99% ee, >99% de[α]D25=+49.9 (c 0.70, CHCl3)Absolute configuration: (7S,8R,2′S,2″S)Source of chirality: (S)-methoxyphenylacetic acid
(7R,8S)-Methyl 7,8-dihydroxytetradecanoateC15H30O4>99% ee[α]D25=+1.7 (c 0.40, CHCl3)Absolute configuration: (7R,8S)Source of chirality: enantioselective HPLC