| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344623 | Tetrahedron: Asymmetry | 2010 | 14 Pages |
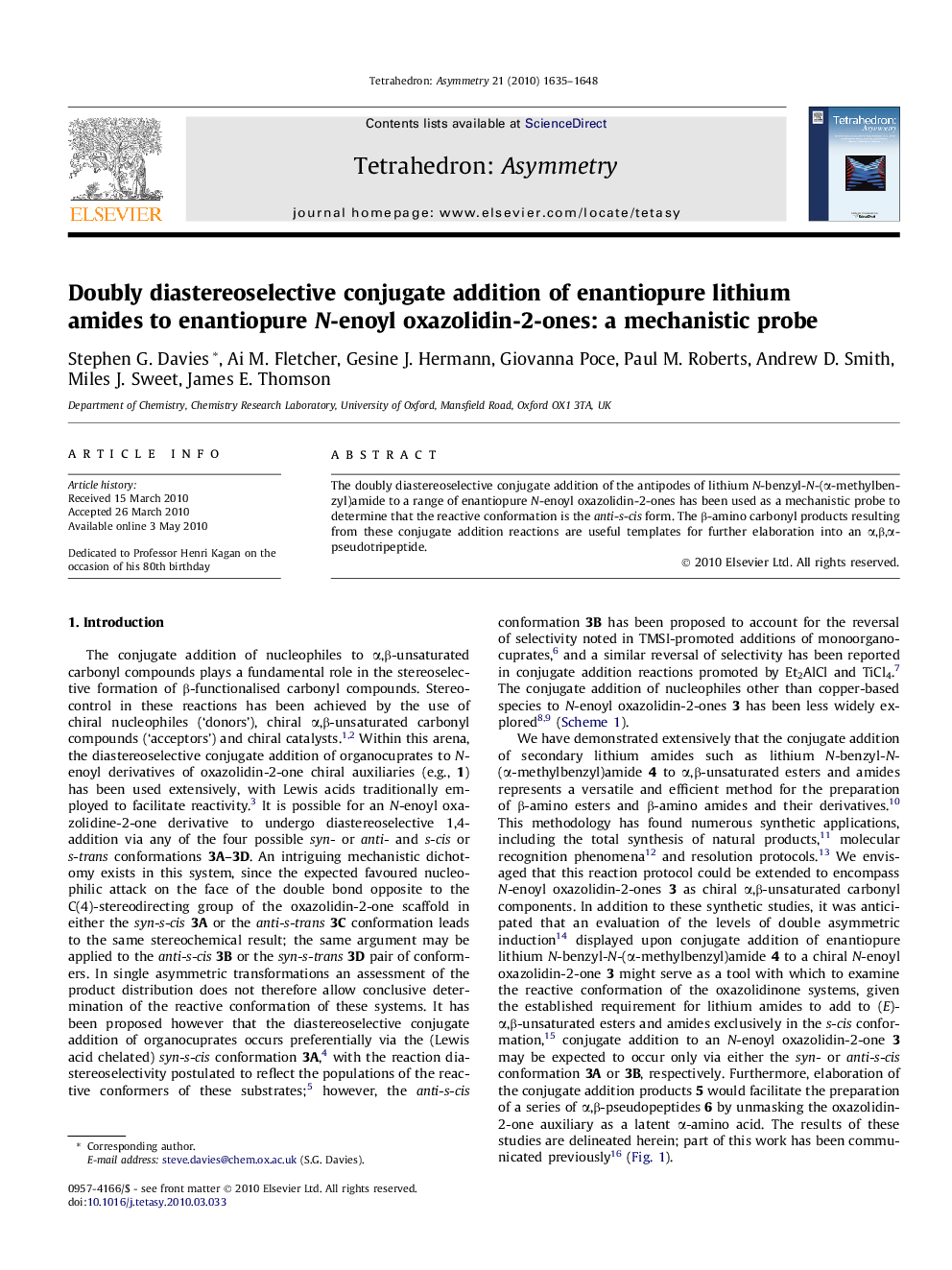
The doubly diastereoselective conjugate addition of the antipodes of lithium N-benzyl-N-(α-methylbenzyl)amide to a range of enantiopure N-enoyl oxazolidin-2-ones has been used as a mechanistic probe to determine that the reactive conformation is the anti-s-cis form. The β-amino carbonyl products resulting from these conjugate addition reactions are useful templates for further elaboration into an α,β,α-pseudotripeptide.
Graphical abstractDoubly diastereoselective conjugate addition of the antipodes of lithium N-benzyl-N-(α-methylbenzyl)amide to N-enoyl oxazolidin-2-ones determines that the reactive conformation is the anti-s-cis form.Figure optionsDownload full-size imageDownload as PowerPoint slide
(4S,3′R,αS)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]-3′-phenylpropanoyl}-4-phenyloxazolidin-2-oneC33H32N2O3[α]D21=+42.7 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R,αS)
(S,S,S)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]butanoyl}-4-phenyloxazolidin-2-oneC28H30N2O3[α]D21=+29.2 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (S,S,S)
(4S,3′R,αS)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]-3′-phenylpropanoyl}-4-benzyloxazolidin-2-oneC34H34N2O3[α]D21=+35.6 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R,αS)
(S,S,S)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]butanoyl}-4-benzyloxazolidin-2-oneC29H32N2O3[α]D21=+6.6 (c 0.9, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (S,S,S)
(4S,3′R,αS)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]-4′-methylpentanoyl}-4-benzyloxazolidin-2-oneC31H36N2O3[α]D20=+10.4 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R,αS)
(4S,3′S,αR)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]-3′-phenylpropanoyl}-4-phenyloxazolidin-2-oneC33H32N2O3[α]D21=+63.5 (c 0.65, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′S,αR)
(4S,3′R,αR)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]butanoyl}-4-phenyloxazolidin-2-oneC28H30N2O3[α]D21=+44.6 (c 1.6, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R,αR)
(4S,3′S,αR)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]-3′-phenylpropanoyl}-4-benzyloxazolidin-2-oneC34H34N2O3[α]D21=+44.2 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′S,αR)
(4S,3′R,αR)-3-{3′-[N-Benzyl-N-(α-methylbenzyl)-amino]butanoyl}-4-benzyloxazolidin-2-oneC29H33N2O3[α]D21=+42.9 (c 0.5, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R,αR)
(4S,3′S,αR)-N(3)-{3′-[N-Benzyl-N-(α-methylbenzyl)amino]-4′-methylpentanoyl}-4-benzyloxazolidin-2-oneC31H36N2O3[α]D20=+58.3 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′S,αR)
(4S,3′R)-N(3)-[3′-(N,N-Dibenzylamino)butanoyl]-4-phenyloxazolidin-2-oneC27H28N2O3[α]D21=+50.3 (c 1.0, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R)
(4S,3′R)-N(3)-[3′-(N,N-Dibenzylamino)-3′-phenylpropanoyl]-4-benzyloxazolidin-2-oneC33H32N2O3[α]D21=+91.4 (c 1.2, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R)
(4S,3′R)-N(3)-(3′-Amino-3′-phenylpropanoyl)-4-benzyloxazolidin-2-oneC19H20N2O3[α]D21=-49.9 (c 2.1, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R)
(4S,3′R,2′′S)-N(3)-(3′-{N′-[2′′-N′′-(tert-Butoxycarbonyl)amino-3′′-phenylpropanoyl]amino}-3′-phenylpropanoyl)-4-benzyloxazolidin-2-oneC33H37N3O6[α]D21=+58.2 (c 1.6, CHCl3)source of chirality: asymmetric synthesisabsolute configuration: (4S,3′R,2′′S)
(S)-N-1′-Hydroxy-3′-phenylpropan-2′-yl (3R,2′′S)-3-{N′-[2′′-N′′-(tert-butoxycarbonyl)amino-3′′-phenylpropanoyl]amino}-3-phenylpropanamideC32H39N3O5[α]D21=-19.1 (c 0.6, DMF)source of chirality: asymmetric synthesisabsolute configuration: (3R,2′S,2′′S)
Boc-[(S)-Phe-(R)-β-Phe-(S)-Phe]-OHC32H37N3O6[α]D23=+13.8 (c 0.5, DMF)source of chirality: asymmetric synthesisabsolute configuration: (S,R,S)
H-[(S)-Phe-(R)-β-Phe-(S)-Phe]-OH trifluoroacetic acid saltC29H29F3N3O5[α]D23=+48.8 (c 0.9, DMF)source of chirality: asymmetric synthesisabsolute configuration: (S,R,S)