| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344632 | Tetrahedron: Asymmetry | 2010 | 7 Pages |
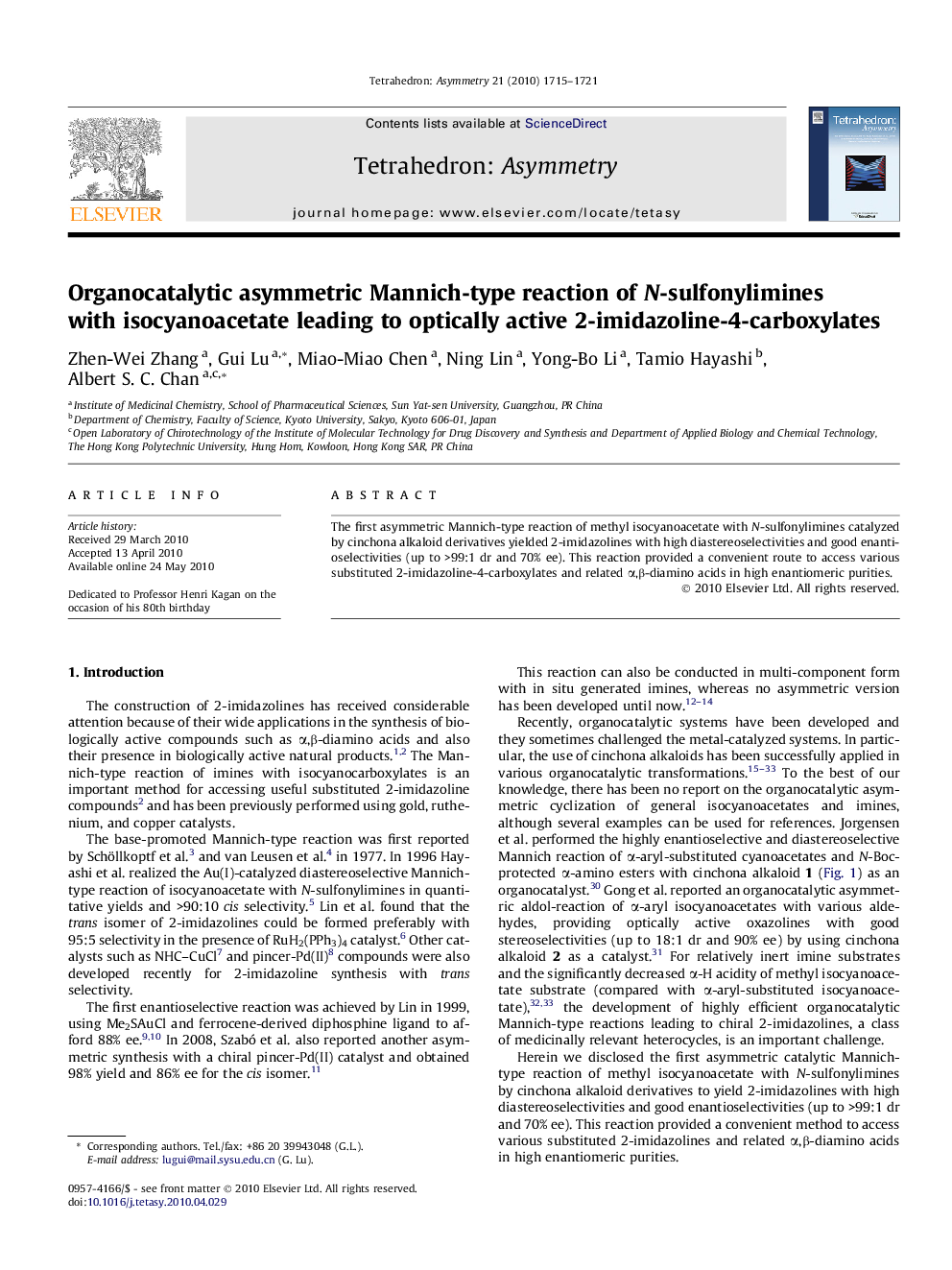
The first asymmetric Mannich-type reaction of methyl isocyanoacetate with N-sulfonylimines catalyzed by cinchona alkaloid derivatives yielded 2-imidazolines with high diastereoselectivities and good enantioselectivities (up to >99:1 dr and 70% ee). This reaction provided a convenient route to access various substituted 2-imidazoline-4-carboxylates and related α,β-diamino acids in high enantiomeric purities.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(4R,5S)-4-(Methoxycarbonyl)-5-(4-methylphenyl)-N-tosyl-2-imidazolineC19H20N2O4SEe = 70%[α]D20=+40 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)
(4R,5S)-4-(Methoxycarbonyl)-5-(4-isopropylphenyl)-N-tosyl-2-imidazolineC21H24N2O4SEe = 62%[α]D20=+35 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)
(4R,5S)-4-(Methoxycarbonyl)-5-(4-tert-butylphenyl)-N-tosyl-2-imidazolineC22H26N2O4SEe = 58%[α]D20=+32 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)
(4R,5S)-4-(Methoxycarbonyl)-5-(4-bromophenyl)-N-tosyl-2-imidazolineC18H17BrN2O4SEe = 48%[α]D20=+20 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)
(4R,5S)-4-(Methoxycarbonyl)-5-(2-methoxyphenyl)-N-tosyl-2-imidazolineC19H20N2O5SEe = 50%[α]D20=+24 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)
(4R,5S)-4-(Methoxycarbonyl)-5-(3-methoxyphenyl)-N-tosyl-2-imidazolineC19H20N2O5SEe = 50%[α]D20=+22 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)
(4R,5S)-4-(Methoxycarbonyl)-5-(2,5-dimethoxyphenyl)-N-tosyl-2-imidazolineC20H22N2O6SEe = 38%[α]D20=+19 (c 1.0, THF)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5S)