| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1344743 | Tetrahedron: Asymmetry | 2010 | 4 Pages |
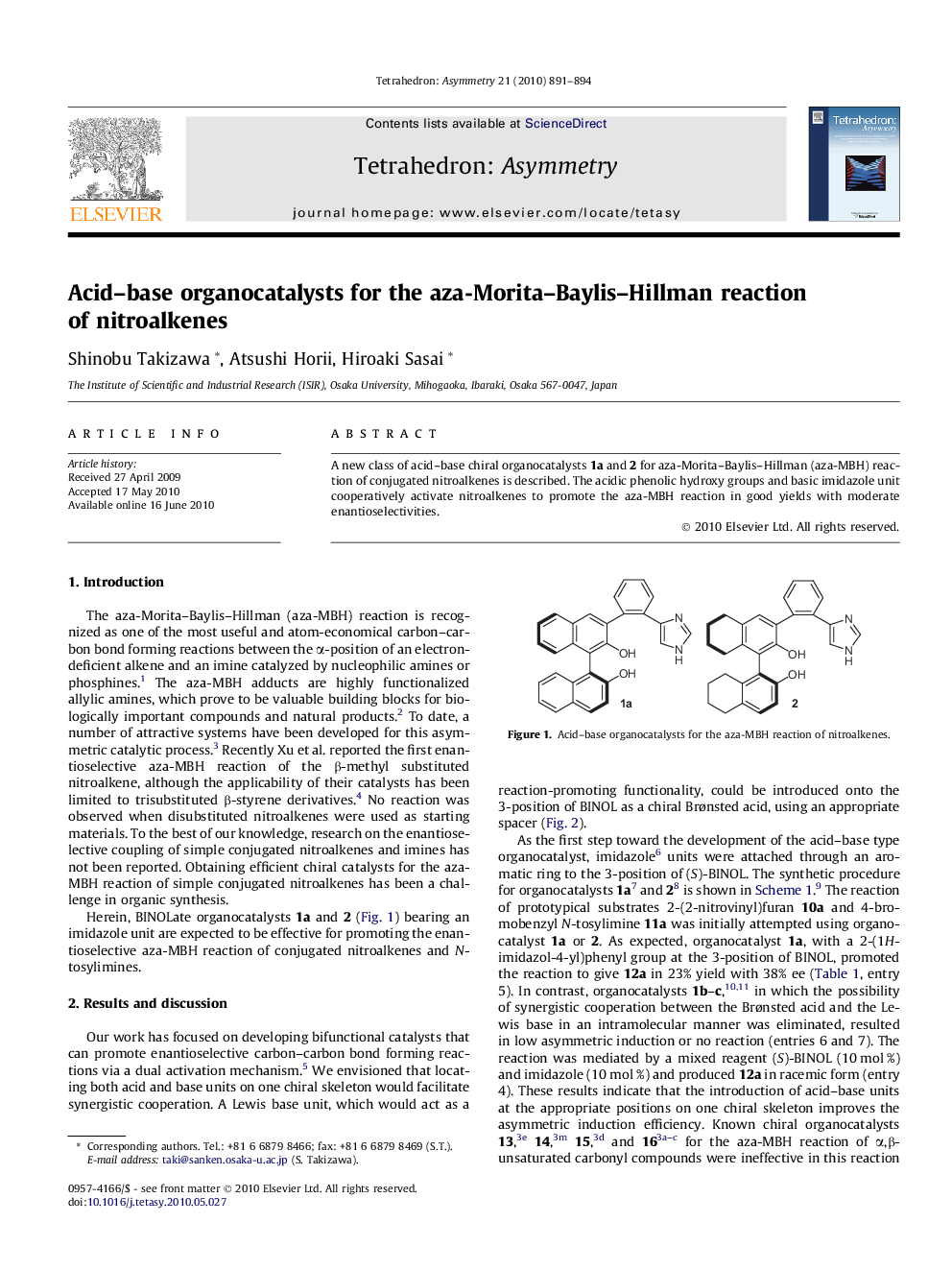
A new class of acid–base chiral organocatalysts 1a and 2 for aza-Morita–Baylis–Hillman (aza-MBH) reaction of conjugated nitroalkenes is described. The acidic phenolic hydroxy groups and basic imidazole unit cooperatively activate nitroalkenes to promote the aza-MBH reaction in good yields with moderate enantioselectivities.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-3-(2-(1H-Imidazol-4-yl)phenyl)-2,2′-dihydroxy-1,1′-binaphthylC29H20N2O2Ee = >99%[α]D = −56.3 (c 0.5, CHCl3)Source of chirality: (S)-BINOLAbsolute configuration: (S)
(S)-3-(2-(1H-Imidazol-4-yl)phenyl)-5,5′,6,6′,7,7′,8,8′-octahydro-2,2′-dihydroxy-1,1′-binaphthylC29H28N2O2Ee = >99%[α]D = −109 (c 0.5, CHCl3)Source of chirality: (S)-BINOLAbsolute configuration: (S)