| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345114 | Tetrahedron: Asymmetry | 2008 | 16 Pages |
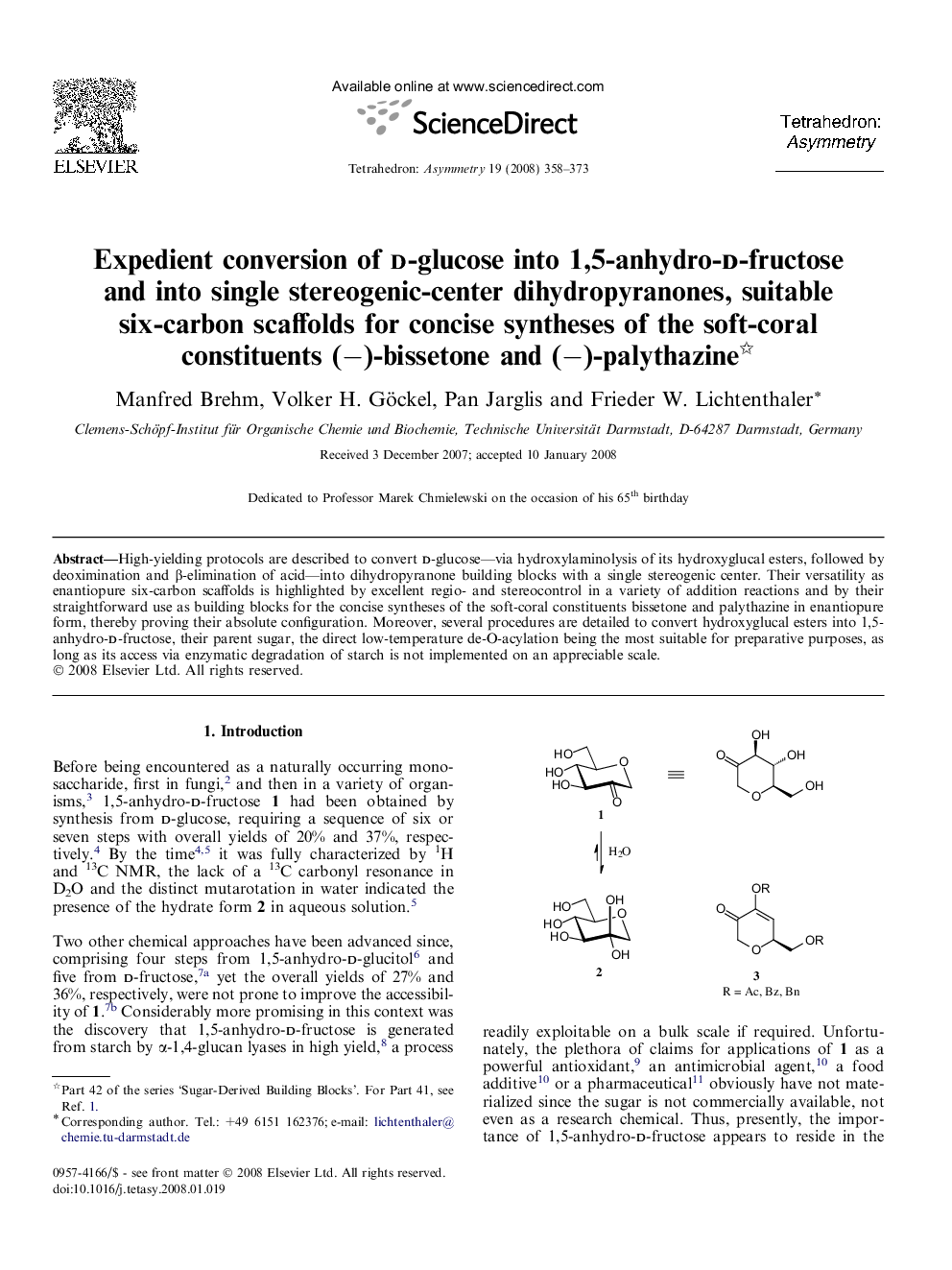
High-yielding protocols are described to convert d-glucose—via hydroxylaminolysis of its hydroxyglucal esters, followed by deoximination and β-elimination of acid—into dihydropyranone building blocks with a single stereogenic center. Their versatility as enantiopure six-carbon scaffolds is highlighted by excellent regio- and stereocontrol in a variety of addition reactions and by their straightforward use as building blocks for the concise syntheses of the soft-coral constituents bissetone and palythazine in enantiopure form, thereby proving their absolute configuration. Moreover, several procedures are detailed to convert hydroxyglucal esters into 1,5-anhydro-d-fructose, their parent sugar, the direct low-temperature de-O-acylation being the most suitable for preparative purposes, as long as its access via enzymatic degradation of starch is not implemented on an appreciable scale.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose semicarbazoneC13H19N3O8[α]D20=-72.5 (c 0.2, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-benzyloximeC19H23NO8[α]D21=-39 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose E-oximeC12H17NO8[α]D20=-42.8 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzoyl-1,5-anhydro-d-fructose E-oximeC27H23NO8[α]D22=-52.9 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzyl-1,5-anhydro-d-fructose E-oximeC27H29NO5[α]D20=-29.0 (c 1.1, CHCl3)Source of chirality: d-glucose
1,5-Anhydro-d-fructose E-oximeC6H11NO5[α]D21=-43.0 (c 0.3, water)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-methyloximeC13H19NO8[α]D21=-29 (c 1, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-acetyloximeC14H19NO9[α]D21=-49 (c 0.3, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructose O-methanesulfonyloximeC13H19NO10S[α]D22=-56.6 (c 0.3, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-acetyl-1,5-anhydro-d-fructoseC12H16O8[α]D21=-10 (c 0.5, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzoyl-1,5-anhydro-d-fructoseC27H22O8[α]D20=-29.2 (c 0.6, CHCl3)Source of chirality: d-glucose
3,4,6-Tri-O-benzyl-1,5-anhydro-d-fructopyranoseC27H28O5[α]D20=-16.1 (c 1.1, CHCl3)Source of chirality: d-glucose
(6S)-4-Benzoyloxy-6-benzoyloxymethyl-2H-pyran-3(6H)-oneC20H16O6[α]D20=-16.0 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-4-Benzyloxy-6-benzyloxymethyl-2H-pyran-3(6H)-oneC20H20O4[α]D20=-32.1 (c 1.1, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
3,4,6-Tri-O-benzoyl-1,5-anhydro-d-fructose diethyldithioacetalC31H32O7S2[α]D23=-51 (c 1.1, CHCl3)Source of chirality: d-glucose
1,5-Anhydro-d-fructose diethyldithioacetalC10H20O4S2[α]D24=-49.8 (c 1, CHCl3)Source of chirality: d-glucose
(6S)-6-Benzoyloxymethyl-3,3-dimethoxy-tetrahydropyran-4-oneC15H18O6[α]D20=-85.6 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-6-Benzoyloxymethyl-3,3-di(benzyloxy)-tetrahydropyran-4-oneC27H26O6[α]D = −94.8 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-4-Benzoyloxy-6-benzoyloxymethyl-2H-pyran-3(6H)-one oximeC20H17NO6[α]D21=-22 (c 0.6, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-4-Benzoyloxy-6-benzoyloxymethyl-2H-pyran-3(6H)-one phenylhydrazoneC26H22N2O5[α]D20=-14 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-6-Benzyloxymethyl-tetrahydropyran-3,4-dione dioximeC13H14N2O5[α]D21=-111 (c 0.5, pyridine)Source of chirality: d-glucoseAbsolute configuration: (6S)
(6S)-6-Benzyloxymethyl-tetrahydropyran-3,4-dione bis(phenylhydrazone)C25H24N4O3[α]D20=-114.3 (c 1.0, CHCl3)[α]D20=-64.3 (c 1.0, pyridine)Source of chirality: d-glucoseAbsolute configuration: (6S)
(3S)-3-Benzyloxymethyl-3,4-dihydro-1H-pyrano[3,4-b]quinoxalineC19H16N2O3[α]D = −81.3 (c 1.1, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (3S)
(2S,4S,5R)-4-Benzoyloxy-2-benzoyloxymethyl-5-hydroxy-tetrahydropyranC20H20O6[α]D25=-18.6 (c 1.0, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,4S,5R)
3,6-Di-O-benzoyl-4-deoxy-4-C-methyl-1,5-anhydro-d-fructoseC21H22O7[α]D20=-6.3 (c 0.5, CHCl3)Source of chirality: d-glucose
(2S,5S)-5-Benzoyloxy-2-benzoyloxymethyl-5-methyl-tetrahydropyran-4-oneC21H20O6[α]D20=-89 (c 1, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,5S)
(2S,5S)-5-Allyl-5-benzoyloxy-2-benzoyloxymethyl-tetrahydropyran-4-oneC23H22O6[α]D20=-65 (c 0.7, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,6S)
(2S)-2-Benzoyloxymethyl-5,5-bis(nitromethyl)-tetrahydropyran-4-oneC15H16N2O8[α]D20=-34.2 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S)
(2S,5S)-2-Benzoyloxymethyl-5-hydroxy-5-acetonyl-tetrahydropyran-4-oneC16H18O6[α]D20=-31.6 (c 1.2, CHCl3)Source of chirality: d-glucoseAbsolute configuration: (2S,5S)
(−)-Bissetone[(2S,5S)-5-acetonyl-5-hydroxy-2-hydroxymethyl-tetrahydropyran-4-one]C9H14O5[α]D20=-69.4 (c 1.2, EtOH)Source of chirality: d-glucoseAbsolute configuration: (2S,5S)
S,S-Palythazine [(3S,8S)-1,3,4,6,8,9-hexahydro-dipyrano[3,4-b:3′,4′-e]pyrazine-3,8-dimethanol]C12H16N2O4[α]D = −198.8 (c 0.35, CH3OH)Source of chirality: d-glucoseAbsolute configuration: (3S,8S)