| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345327 | Tetrahedron: Asymmetry | 2015 | 10 Pages |
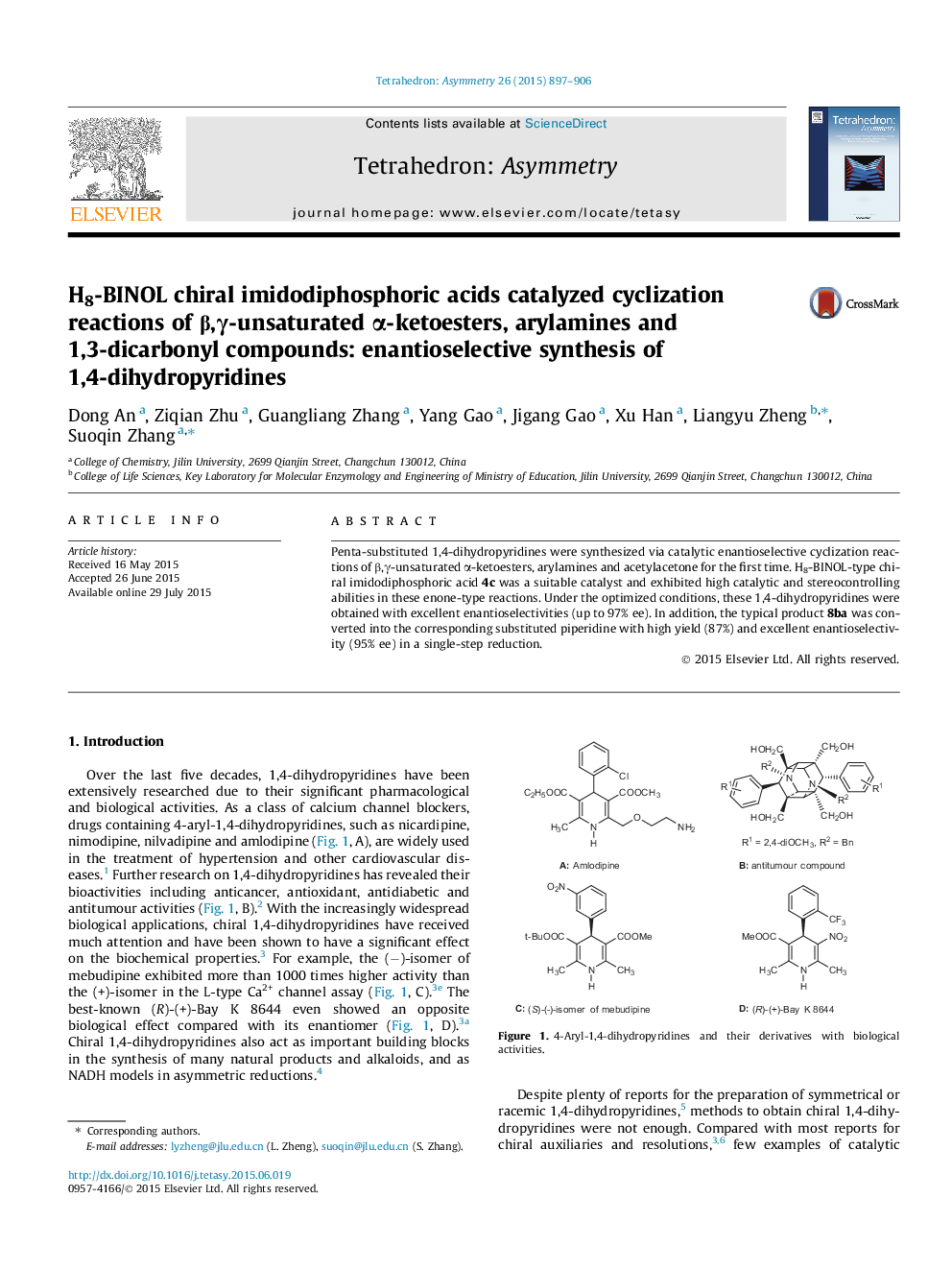
Penta-substituted 1,4-dihydropyridines were synthesized via catalytic enantioselective cyclization reactions of β,γ-unsaturated α-ketoesters, arylamines and acetylacetone for the first time. H8-BINOL-type chiral imidodiphosphoric acid 4c was a suitable catalyst and exhibited high catalytic and stereocontrolling abilities in these enone-type reactions. Under the optimized conditions, these 1,4-dihydropyridines were obtained with excellent enantioselectivities (up to 97% ee). In addition, the typical product 8ba was converted into the corresponding substituted piperidine with high yield (87%) and excellent enantioselectivity (95% ee) in a single-step reduction.
Graphical abstractThe H8-BINOL chiral imidodiphosphoric acids catalyzed enantioselective cyclization reaction of β,γ-unsaturated α-ketoesters, arylamines and acetylacetone was developed. Five-substituted 4-aryl-1,4-dihydropyridines were obtained with up to 61% yields and up to 97% ee.Figure optionsDownload full-size imageDownload as PowerPoint slide
(R)-Methyl-5-acetyl-1-(3-methoxyphenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC23H22N2O688% ee[α]D20 = +143.4 (c 0.456, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Ethyl-5-acetyl-1-(3-methoxyphenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC24H24N2O685% ee[α]D25 = +145.2 (c 0.566, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-methoxyphenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC25H26N2O690% ee[α]D25 = +238.0 (c 0.484, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Benzyl-5-acetyl-1-(3-methoxyphenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC29H26N2O689% ee[α]D25 = +179.8 (c 0.502, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-chlorophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC24H23ClN2O591% ee[α]D25 = +226.7 (c 0.486, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(4-chlorophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC24H23ClN2O589% ee[α]D25 = +226.5 (c 0.476, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-bromophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC24H23BrN2O588% ee[α]D25 = +240.6 (c 0.271, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-fluorophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC24H23FN2O594% ee[α]D25 = +248.0 (c 0.508, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(4-fluorophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC24H23FN2O587% ee[α]D25 = +303.4 (c 0.470, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-6-methyl-4-(3-nitrophenyl)-1-(m-tolyl)-1,4-dihydropyridine-2-carboxylateC25H26N2O583% ee[α]D25 = +117.4 (c 0.301, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-6-methyl-4-(3-nitrophenyl)-1-(p-tolyl)-1,4-dihydropyridine-2-carboxylateC25H26N2O575% ee[α]D25 = +102.2 (c 0.256, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Benzyl-5-acetyl-1-(3-chlorophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC28H23ClN2O594% ee[α]D25 = +189.2 (c 0.446, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Benzyl-5-acetyl-1-(3-bromophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC28H23BrN2O594% ee[α]D25 = +193.5 (c 0.307, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-4-(4-bromophenyl)-1-(3-chlorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC24H23BrClNO392% ee[α]D25 = +417.2 (c 0.494, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-bromophenyl)-4-(4-bromophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC24H23Br2NO391% ee[α]D25 = +160.9 (c 0.307, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Methyl-5-acetyl-4-(3-bromophenyl)-1-(3-chlorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC22H19BrClNO393% ee[α]D25 = +217 (c 0.203, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-chlorophenyl)-4-(4-cyanophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC25H23ClN2O395% ee[α]D25 = +704 (c 0.486, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-4-(4-cyanophenyl)-1-(3-fluorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC25H23FN2O394% ee[α]D25 = +249.9 (c 0.214, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Ethyl-5-acetyl-1-(3-bromophenyl)-4-(4-fluorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC23H21BrFNO391% ee[α]D25 = +213.1 (c 0.502, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Ethyl-5-acetyl-1-(3-chlorophenyl)-6-methyl-4-(4-(trifluoromethyl)phenyl)-1,4-dihydropyridine-2-carboxylateC24H21ClF3NO391% ee[α]D25 = +205.7 (c 0.244, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Methyl-5-acetyl-1-(3-chlorophenyl)-4-(3,4-dichlorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC22H18Cl3NO395% ee[α]D25 = +240.0 (c 0.490, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-chlorophenyl)-4-(3,4-dichlorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC24H22Cl3NO393% ee[α]D25 = +191.6 (c 0.262, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Methyl-5-acetyl-1-(3-bromophenyl)-4-(3,4-dichlorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC22H18BrCl2NO394% ee[α]D25 = +201.5 (c 0.271, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Ethyl-5-acetyl-1-(3-bromophenyl)-4-(3,4-dichlorophenyl)-6-methyl-1,4-dihydropyridine-2-carboxylateC23H20BrCl2NO392% ee[α]D25 = +179.5 (c 0.507, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Isopropyl-5-acetyl-1-(3-bromophenyl)-6-methyl-4-(p-tolyl)-1,4-dihydropyridine-2-carboxylateC25H26BrNO390% ee[α]D25 = +195.6 (c 0.226, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Methyl-5-acetyl-1-(3-bromophenyl)-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC22H19BrN2O594% ee[α]D25 = +273.4 (c 0.237, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(R)-Benzyl-5-acetyl-1-(3-fluorophenyl)-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridine-2-carboxylateC28H23FN2O597% ee[α]D25 = +251.5 (c 0.334, EtOAc)Source of chirality: asymmetric synthesisAbsolute configuration: (R)