| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345584 | Tetrahedron: Asymmetry | 2013 | 6 Pages |
The synthesis of chemically and enantiomerically pure (S)-3-amino tetrahydrofuran hydrochloride starting from the natural amino acids, l-aspartic acid or l-methionine is described. The process involves no chromatography and can be easily carried out on a large scale. The enantiopurity of the final product was established by NMR and chiral HPLC methods.
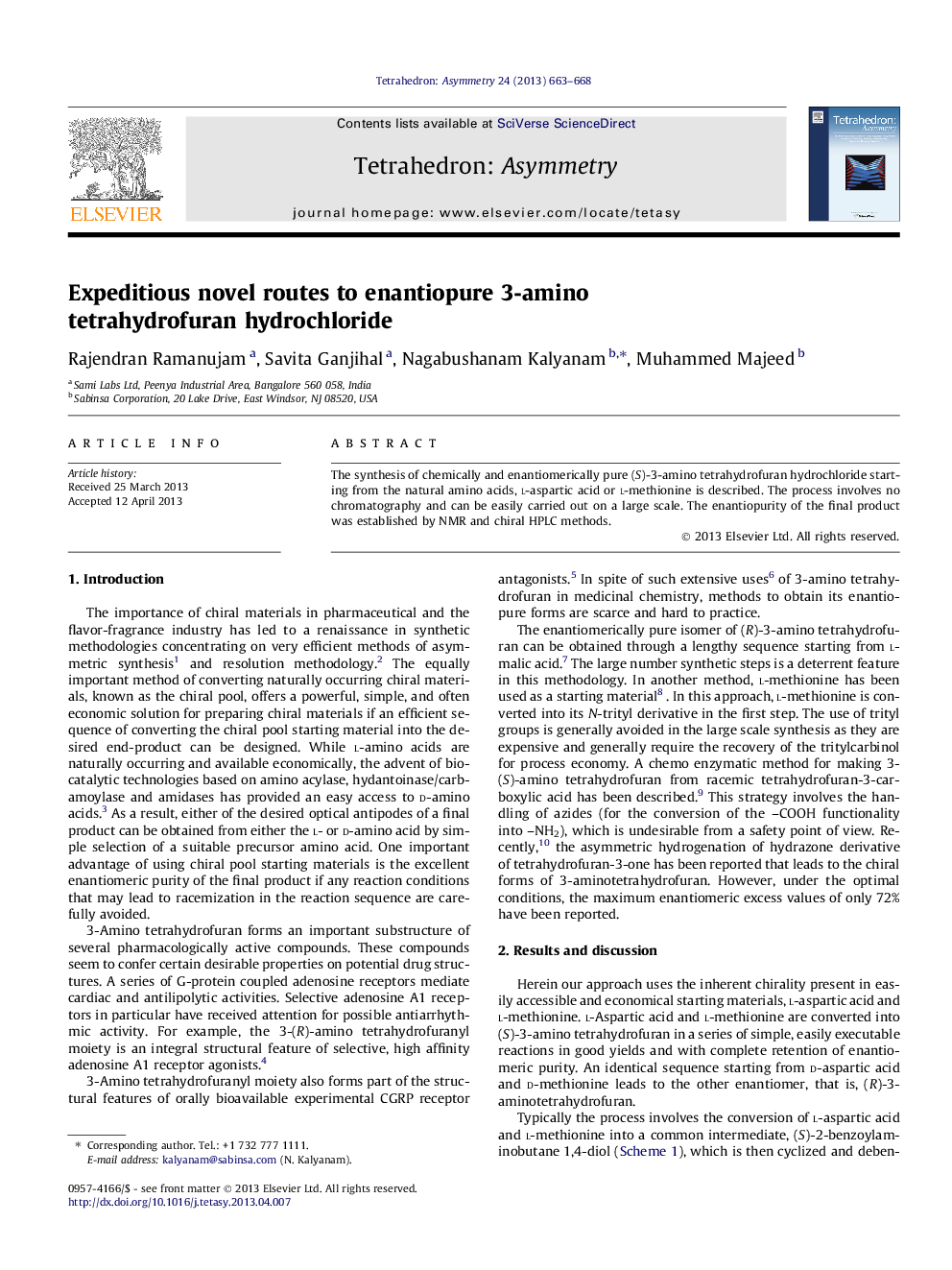
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-3-Aminotetrahydrofuran hydrochlorideC4H10ClNOee ⩾99%[α]D27 = −10.2 (c 1.0, methanol)Source of chirality: l-aspartic acid/l-methionineAbsolute configuration: (S)
(S)-2-Benzoylamino gamma butyrolactoneC11H11NO3ee ⩾99%[α]D27 = −27.8 (c 1.0, ethanol)Source of chirality: l-methionineAbsolute configuration: (S)
(S)-3-Benzoylamino tetrahydrofuranC11H13NO2ee ⩾99%[α]D27 = −29.4 (c 1.0, methanol)Source of chirality: l-aspartic acid/l-methionineAbsolute configuration: (S)
(S)-2-Benzoylaminobutane 1,4 diolC11H15NO3ee ⩾99%[α]D27 = −39.7 (c 0.05, methanol)Source of chirality: l-aspartic acid/l-methionineAbsolute configuration: (S)