| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345629 | Tetrahedron: Asymmetry | 2006 | 4 Pages |
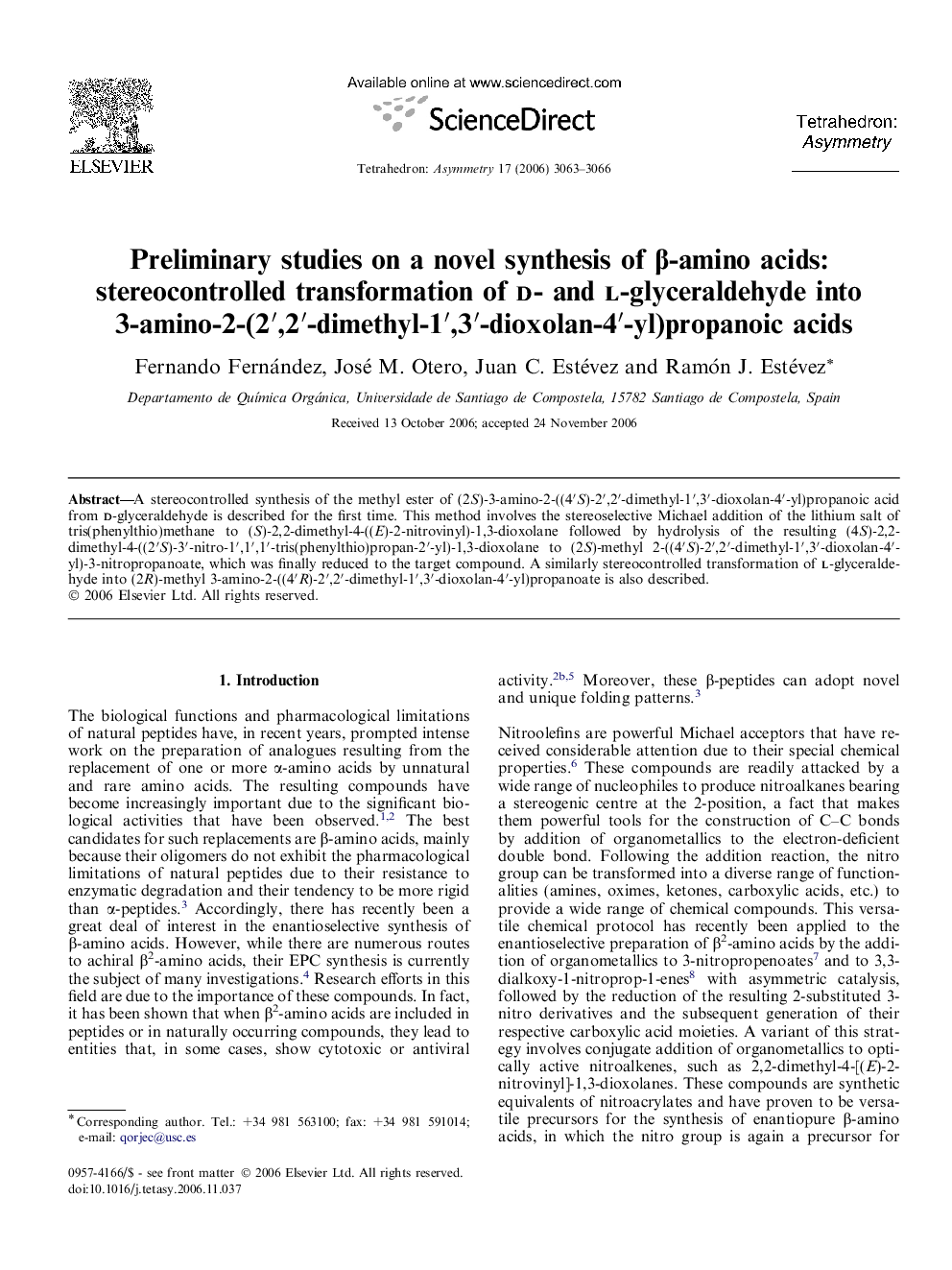
A stereocontrolled synthesis of the methyl ester of (2S)-3-amino-2-((4′S)-2′,2′-dimethyl-1′,3′-dioxolan-4′-yl)propanoic acid from d-glyceraldehyde is described for the first time. This method involves the stereoselective Michael addition of the lithium salt of tris(phenylthio)methane to (S)-2,2-dimethyl-4-((E)-2-nitrovinyl)-1,3-dioxolane followed by hydrolysis of the resulting (4S)-2,2-dimethyl-4-((2′S)-3′-nitro-1′,1′,1′-tris(phenylthio)propan-2′-yl)-1,3-dioxolane to (2S)-methyl 2-((4′S)-2′,2′-dimethyl-1′,3′-dioxolan-4′-yl)-3-nitropropanoate, which was finally reduced to the target compound. A similarly stereocontrolled transformation of l-glyceraldehyde into (2R)-methyl 3-amino-2-((4′R)-2′,2′-dimethyl-1′,3′-dioxolan-4′-yl)propanoate is also described.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(4R)-2,2-Dimethyl-4-((E)-2-nitrovinyl)-1,3-dioxolaneC7H11NO4[α]D27=-37.8 (c 1.05, CHCl3)Source of asymmetry: l-ascorbic acidAbsolute configuration: (4R)
(4S)-2,2-Dimethyl-4-((2′S)-3′-nitro-1′,1′,1′-tris(phenylthio)propan-2′-yl)-1,3-dioxolaneC26H27NO4S3[α]D27=-44.5 (c 1.05, CHCl3)Source of asymmetry: d-mannitolAbsolute configuration: (4S,2′S)
Methyl (2S)-2-((4′S)-2′,2′-dimethyl-1′,3′-dioxolan-4′-yl)-3-((N-(benzyloxycarbonyl)glycyl)amino)propanoateC19H26N2O7[α]D27=-14.7 (c 1.20, CHCl3)Source of asymmetry: d-mannitolAbsolute configuration: (2S,4′S)
(2S)-Methyl 2-((4′S)-2′,2′-dimethyl-1′,3′-dioxolan-4′-yl)-3-nitropropanoateC9H15NO6[α]D26=-23.2 (c 1.23, CHCl3)Source of asymmetry: d-mannitolAbsolute configuration: (2S,4′S)
Methyl N-((2S)-2-((4′S)-2′,2′-dimethyl-1′,3′-dioxolan-4′-yl)-3-((N-(methoxycarbonyl)glycyl)amino)propanoyl)glycinateC21H29N3O8[α]D27=-15.8 (c 2.30, CHCl3)Source of asymmetry: d-mannitolAbsolute configuration: (2S,4′S)