| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345718 | Tetrahedron: Asymmetry | 2016 | 8 Pages |
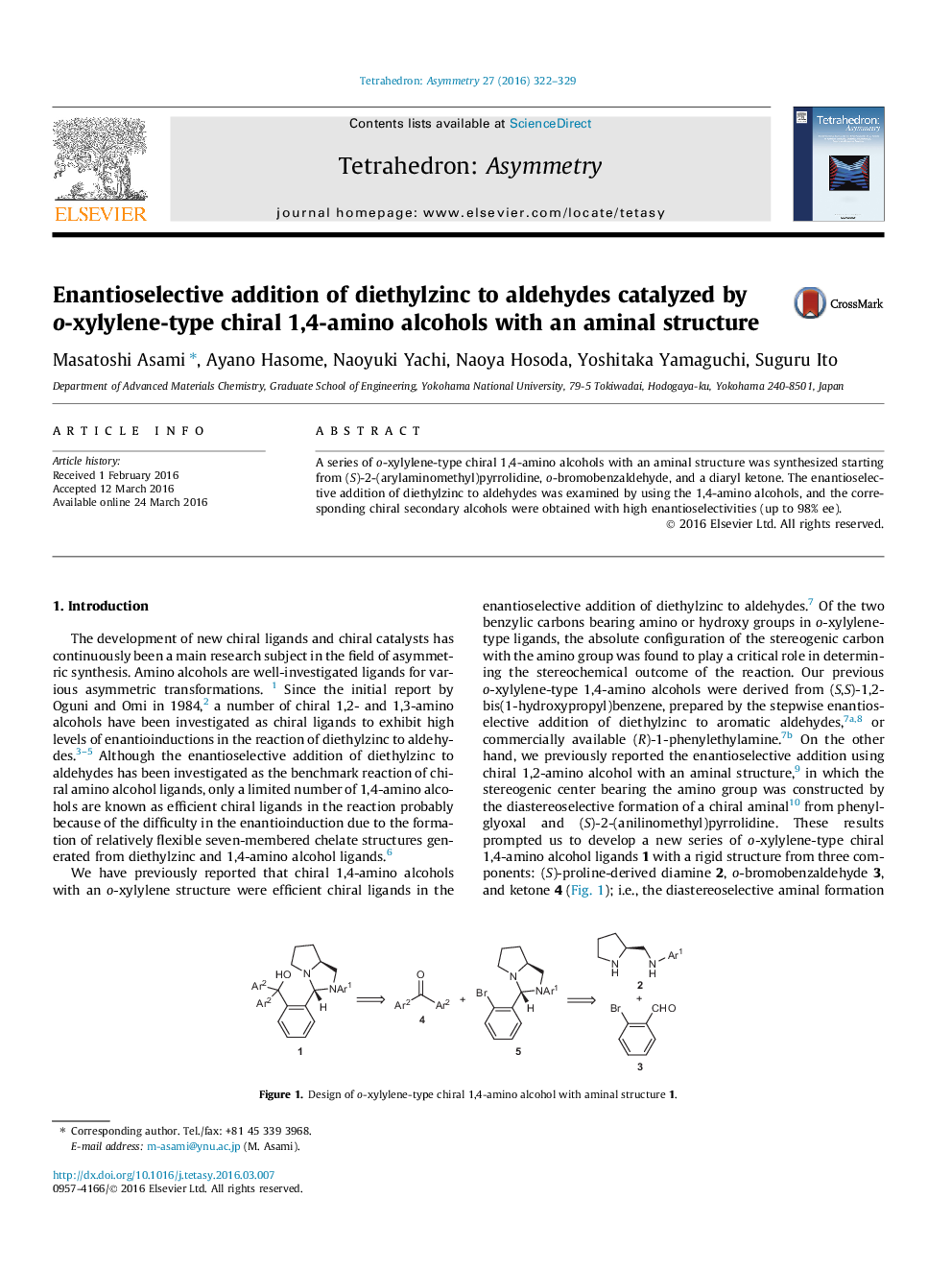
A series of o-xylylene-type chiral 1,4-amino alcohols with an aminal structure was synthesized starting from (S)-2-(arylaminomethyl)pyrrolidine, o-bromobenzaldehyde, and a diaryl ketone. The enantioselective addition of diethylzinc to aldehydes was examined by using the 1,4-amino alcohols, and the corresponding chiral secondary alcohols were obtained with high enantioselectivities (up to 98% ee).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(3R,7aS)-3-(2-Bromophenyl)-2-phenylhexahydro-1H-pyrrolo[1,2-c]imidazoleC18H19BrN2[α]D23=+124 (c 1.0, CH2Cl2)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(3R,7aS)-3-(2-Bromophenyl)-2-(4-methoxyphenyl)hexahydro-1H-pyrrolo[1,2-c]imidazoleC19H21BrN2O[α]D19=+96.2 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(3R,7aS)-3-(2-Bromophenyl)-2-(4-(trifluoromethyl)phenyl)hexahydro-1H-pyrrolo[1,2-c]imidazoleC19H18BrF3N2[α]D19=+86.7 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
Diphenyl(2-((3R,7aS)-2-phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)methanolC31H30N2O[α]D28=+292.9 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(2-((3R,7aS)-2-(4-Methoxyphenyl)hexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)diphenylmethanolC32H32N2O2[α]D23=+210.4 (c 1.1, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
Diphenyl(2-((3R,7aS)-2-(4-(trifluoromethyl)phenyl)hexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)methanolC32H29F3N2O[α]D23=+227.0 (c 1.3, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(2-((3R,7aS)-2-Phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)di-p-tolylmethanolC33H34N2O[α]D28=+281.0 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
Bis(4-methoxyphenyl)(2-((3R,7aS)-2-phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)methanolC33H34N2O3[α]D23=+281.7 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(2-((3R,7aS)-2-Phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)bis(4-(trifluoromethyl)phenyl)methanolC33H28F6N2O[α]D28=+241.6 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(2-((3R,7aS)-2-Phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)bis(3-(trifluoromethyl)phenyl)methanolC33H28F6N2O[α]D28=+253.3 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(2-((3R,7aS)-2-Phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)bis(2-(trifluoromethyl)phenyl)methanolC33H28F6N2O[α]D28=+199.8 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
Bis(3,5-bis(trifluoromethyl)phenyl)(2-((3R,7aS)-2-phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)methanolC35H26F12N2O[α]D28=+205.0 (c 0.47, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
(2-((3R,7aS)-2-Phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)bis(3,4,5-trifluorophenyl)methanolC31H24F6N2O[α]D21=+215.0 (c 1.0, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)
Bis(perfluorophenyl)(2-((3R,7aS)-2-phenylhexahydro-1H-pyrrolo[1,2-c]imidazol-3-yl)phenyl)methanolC31H20F10N2O[α]D23=+144.0 (c 0.94, CHCl3)Source of chirality: l-prolineAbsolute configuration: (3R,7aS)