| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345775 | Tetrahedron: Asymmetry | 2013 | 4 Pages |
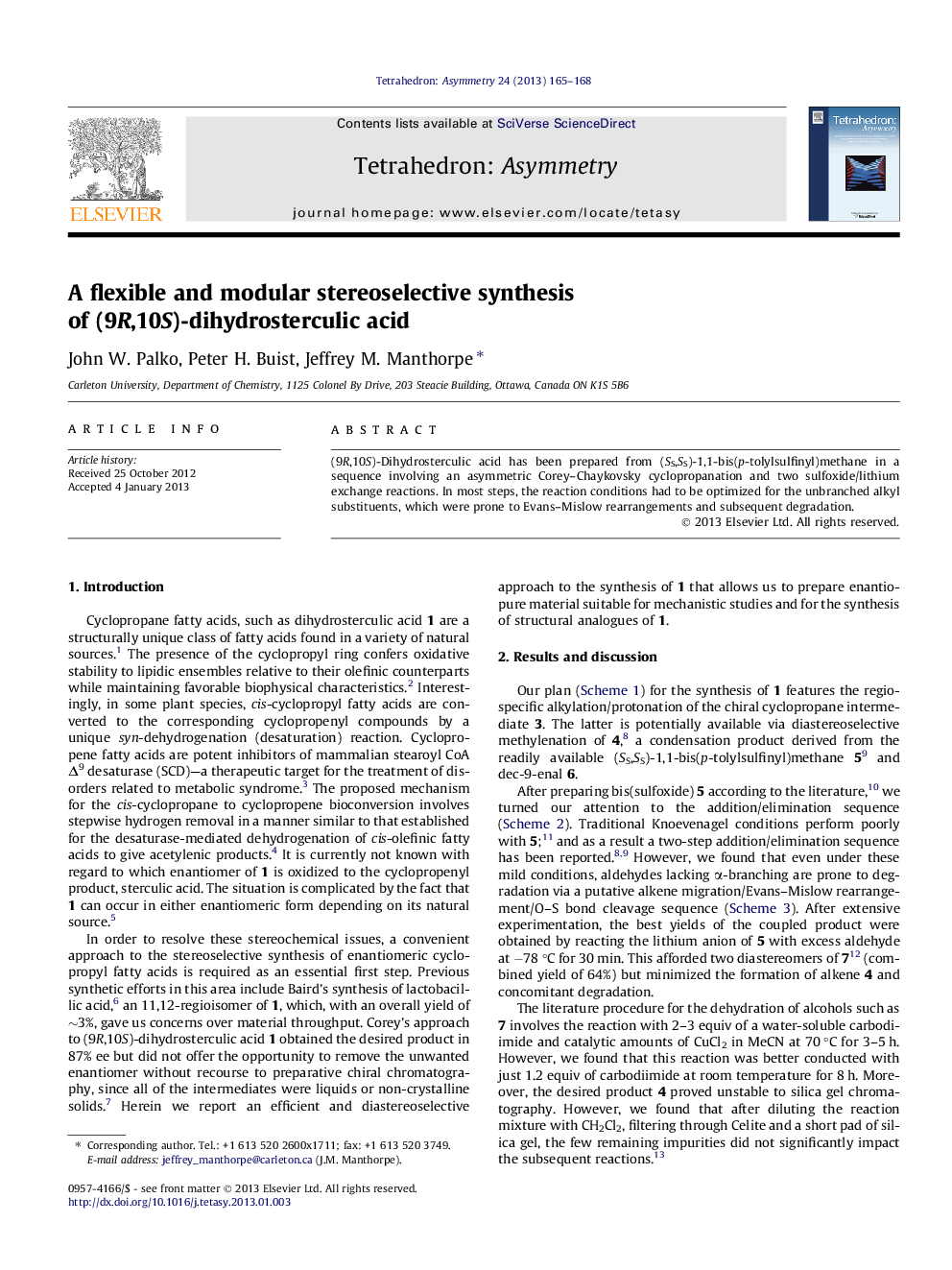
(9R,10S)-Dihydrosterculic acid has been prepared from (SS,SS)-1,1-bis(p-tolylsulfinyl)methane in a sequence involving an asymmetric Corey–Chaykovsky cyclopropanation and two sulfoxide/lithium exchange reactions. In most steps, the reaction conditions had to be optimized for the unbranched alkyl substituents, which were prone to Evans–Mislow rearrangements and subsequent degradation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1R,2S)-0031-(Non-8-enyl)-2-n-octylcyclopropaneC20H38[α]D22=+2.5 (c 0.393, CHCl3)Source of chirality: (1R,2S,5R)-(−)-mentholAbsolute configuration: (1R,2S)
1,1-Bis[(SS)-p-toluenesulfinyl]-2-(non-8-enyl)cyclopropaneC26H34O2S2[α]D22=-113.3 (c 0.233, CHCl3)Source of chirality: (1R,2S,5R)-(−)-mentholAbsolute configuration: (SS,SS,2R)
1-n-Octyl-1-[(SS)-p-toluenesulfinyl]-2-(non-8-enyl)cyclopropaneC27H44OS[α]D22=+54.2 (c 0.273, CHCl3)Source of chirality: (1R,2S,5R)-(−)-mentholAbsolute configuration: (SS,1R,2R)
Methyl (9R,10S)-dihydrosterculate/methyl 8-((1R,2S)-2-octylcyclopropyl)octanoateC20H38O2[α]D22=+1.7 (c 0.573, CHCl3)Source of chirality: (1R,2S,5R)-(−)-mentholAbsolute configuration: (9R,10S)