| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345778 | Tetrahedron: Asymmetry | 2013 | 6 Pages |
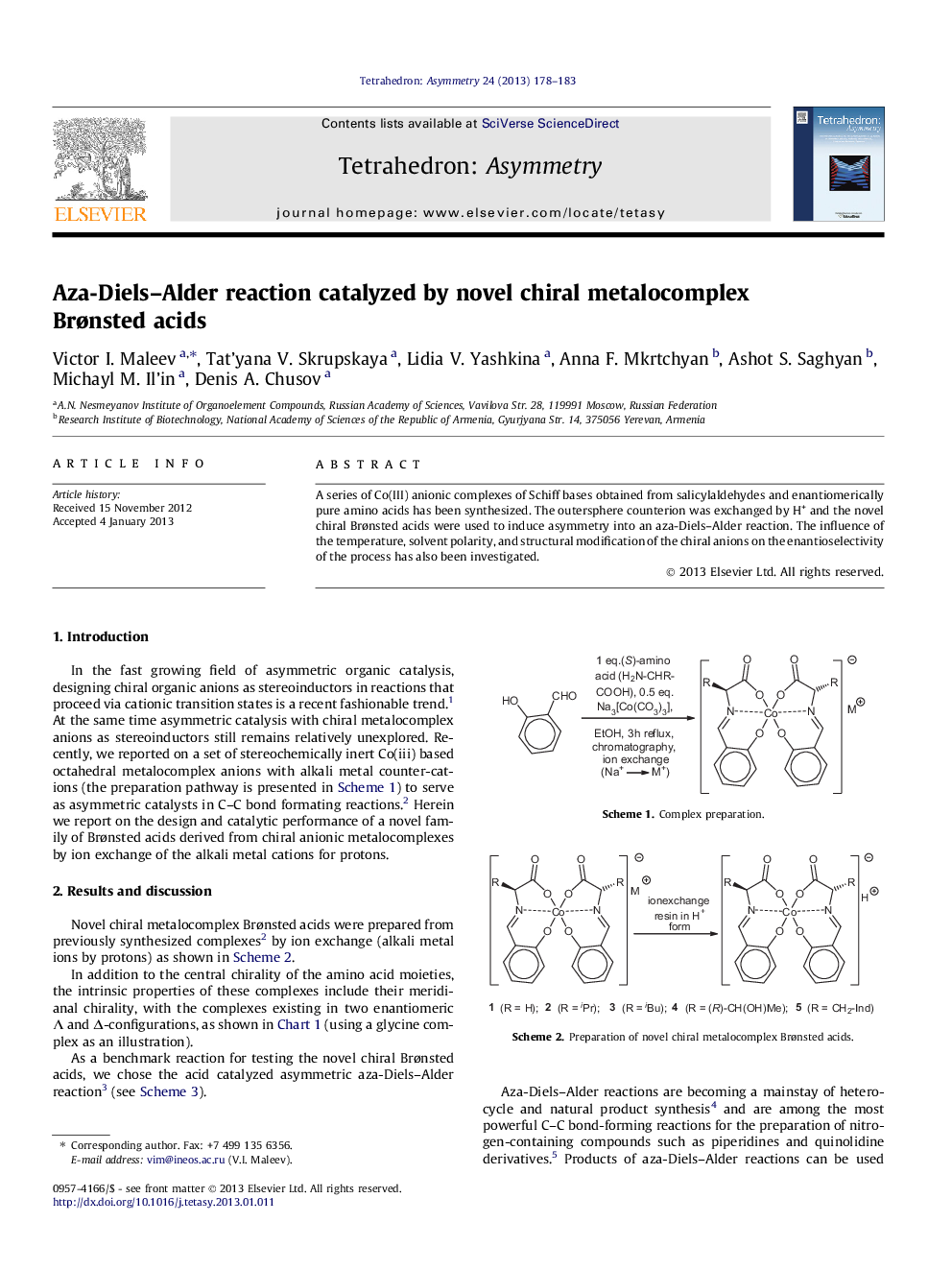
A series of Co(III) anionic complexes of Schiff bases obtained from salicylaldehydes and enantiomerically pure amino acids has been synthesized. The outersphere counterion was exchanged by H+ and the novel chiral Brønsted acids were used to induce asymmetry into an aza-Diels–Alder reaction. The influence of the temperature, solvent polarity, and structural modification of the chiral anions on the enantioselectivity of the process has also been investigated.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Hydrogen Δ-[bis(N-salicylidene-(S)-leucinato)cobaltate(iii)]Ee >99%[α]D25=-5692 (c 0.05, MeOH)Source of chirality: synthesis from (S)-leucineAbsolute configuration: (Δ,S,S)
(1S,3R,4S)-2,3-Diphenyl-2-azabicyclo[2.2.2]octan-5-oneEe 98%[α]D25=+74.6 (c 0.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
(1S,3R,4S)-3-(4-Methoxyphenyl)-2-phenyl-2-azabicyclo[2.2.2]octan-5-oneEe 94%[α]D25=+36.4 (c 0.11, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
(1S,3R,4S)-3-(3-Chlorophenyl)-2-phenyl-2-azabicyclo[2.2.2]octan-5-oneEe 71%[α]D25=+50.8 (c 0.33, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
(1S,3R,4S)-2-(4-Chlorophenyl)-3-phenyl-2-azabicyclo[2.2.2]octan-5-oneEe 76%[α]D25=+53.6 (c 0.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)