| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345965 | Tetrahedron: Asymmetry | 2012 | 10 Pages |
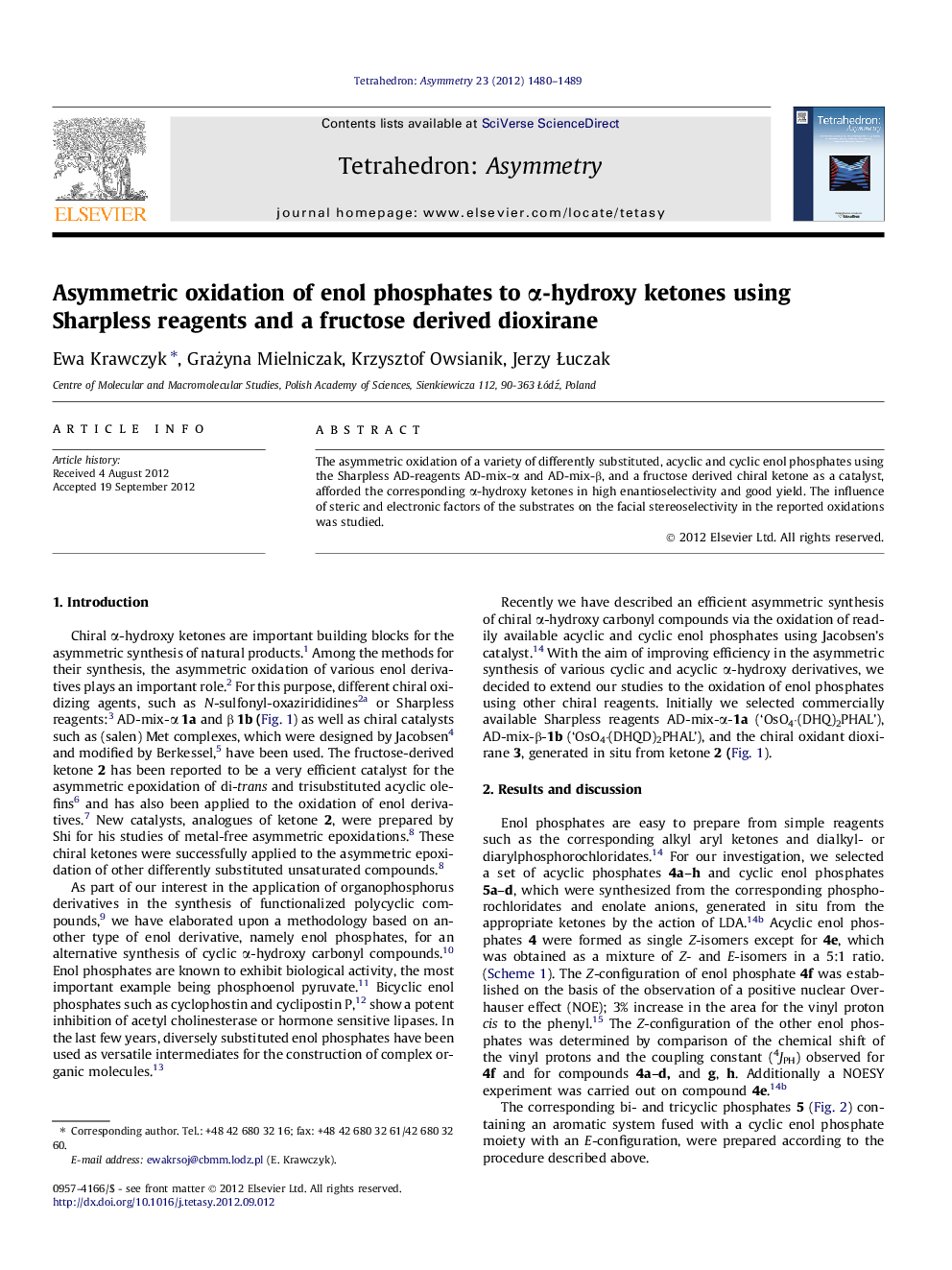
The asymmetric oxidation of a variety of differently substituted, acyclic and cyclic enol phosphates using the Sharpless AD-reagents AD-mix-α and AD-mix-β, and a fructose derived chiral ketone as a catalyst, afforded the corresponding α-hydroxy ketones in high enantioselectivity and good yield. The influence of steric and electronic factors of the substrates on the facial stereoselectivity in the reported oxidations was studied.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(2S)-2-Hydroxy-1-phenyl-1-propan-1-oneC9H10O2ee = 98.6% (HPLC)[α]D20=-96.4 (c 0.7, CHCl3)Source of chirality: asymmetric synthesis by AD-mix-αAbsolute configuration (S)
(2S)-2-Hydroxy-1-(4-methoxy-phenyl)-1-propan-1-oneC10H12O3ee = 100% (HPLC)[α]D20=-79 (c 0.7, CHCl3)Source of chirality: asymmetric synthesis by AD-mix-αAbsolute configuration (S)
(2S)-2-Hydroxy-1-phenyl-1-butan-1-oneC10H12O2ee = 92% (HPLC)[α]D20=-34.4 (c 0.7, CHCl3)Source of chirality: asymmetric synthesis by AD-mix-αAbsolute configuration (S)
(2R)-2-Hydroxy-1,2-diphenyl-ethanoneC14H12O2ee = 100% (HPLC)[α]D20=-142 (c 0.7, CHCl3)Source of chirality: asymmetric synthesis by AD-mix-βAbsolute configuration (R)
(2R)-2-Hydroxy-3,4-dihydro-2H-naphthalen-1-oneC10H10O2ee = 88% (HPLC)[α]D20=+14.7 (c 0.6, CHCl3)Source of chirality: asymmetric synthesis with a chiral dioxiraneAbsolute configuration (R)
(2R)-3-Hydroxy-2,3-dihydro-4H-chromen-4-oneC9H8O3ee = 92% (HPLC)[α]D20=+82.7 (c 0.85, CHCl3)Source of chirality: asymmetric synthesis with a chiral dioxiraneAbsolute configuration (R)
(3S)-3-Hydroxy-2,3-dihydro-1H-phenanthren-4-oneC14H12O2ee = 84% (HPLC)[α]D20=-20.7 (c 0.8, CHCl3)Source of chirality: asymmetric synthesis with a chiral dioxiraneAbsolute configuration (S)
(2S)-2-Hydroxy-2-methyl-1-indanoneC10H10O2ee = 12% (HPLC)[α]D20=-3.7 (c 0.4, CHCl3)Source of chirality: asymmetric synthesis with a chiral dioxiraneAbsolute configuration (S)
(2S)-2-Hydroxy-1-phenyl-1-pentan-1-oneC11H14O2Ee = 76% (HPLC)[α]D20=-10.2 (c 0.14, CHCl3)Source of chirality: asymmetric synthesis with a chiral dioxiraneAbsolute configuration (S)