| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345982 | Tetrahedron: Asymmetry | 2012 | 4 Pages |
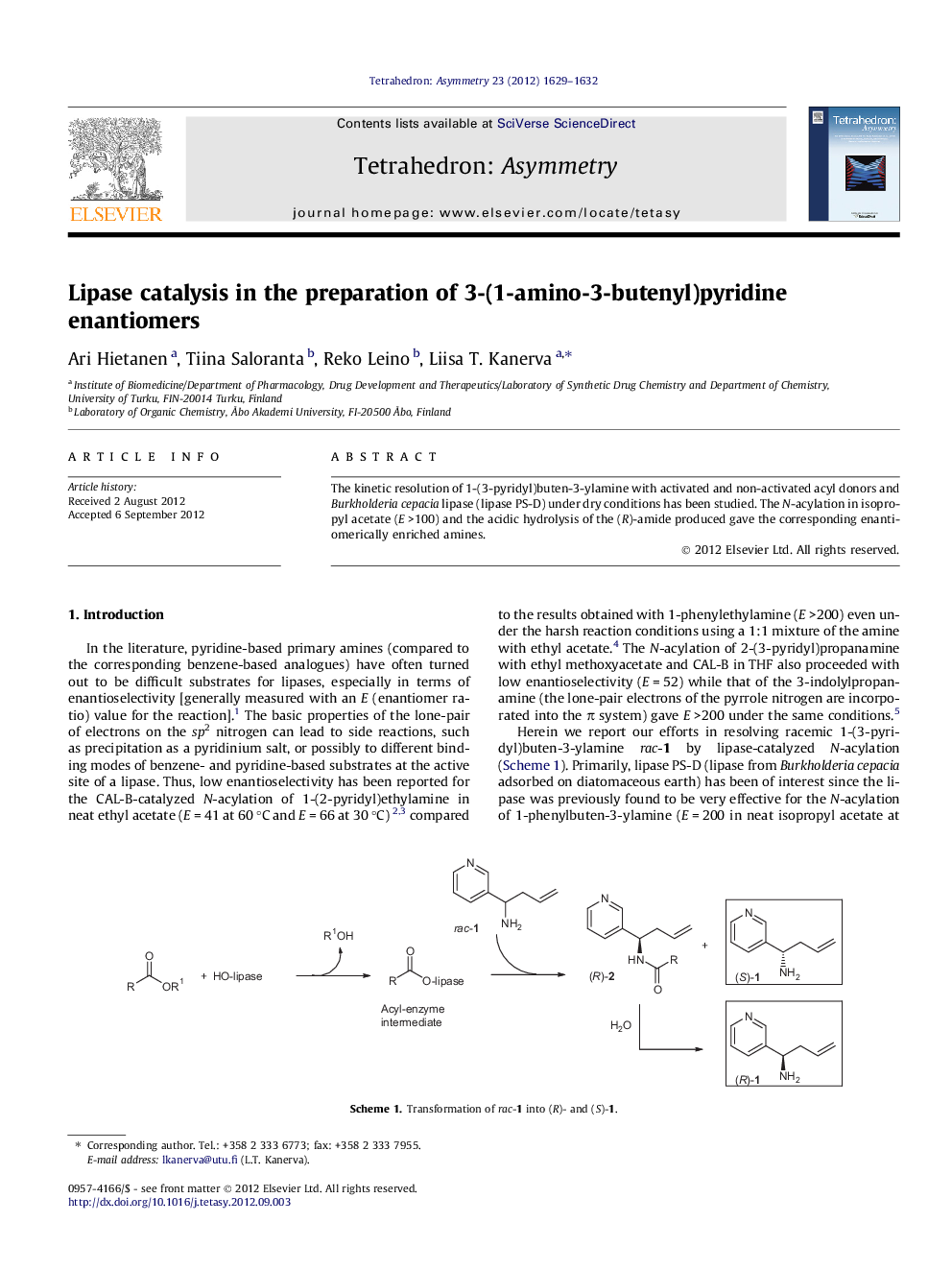
The kinetic resolution of 1-(3-pyridyl)buten-3-ylamine with activated and non-activated acyl donors and Burkholderia cepacia lipase (lipase PS-D) under dry conditions has been studied. The N-acylation in isopropyl acetate (E >100) and the acidic hydrolysis of the (R)-amide produced gave the corresponding enantiomerically enriched amines.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(R)-1-(3-Pyridyl)buten-3-ylacetamideC11H14N2OEe = 97%[α]D25=+82 (c 1, CHCl3)Source of chirality: enzymatic resolution by Burkholderia cepacia lipaseAbsolute configuration: (R)
(S)-1-(3-Pyridyl)buten-3-ylamineC9H12N2Ee = 88%[α]D25=-39 (c 1, CHCl3)Source of chirality: enzymatic resolution by Burkholderia cepacia lipaseAbsolute configuration: (S)