| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1345990 | Tetrahedron: Asymmetry | 2012 | 5 Pages |
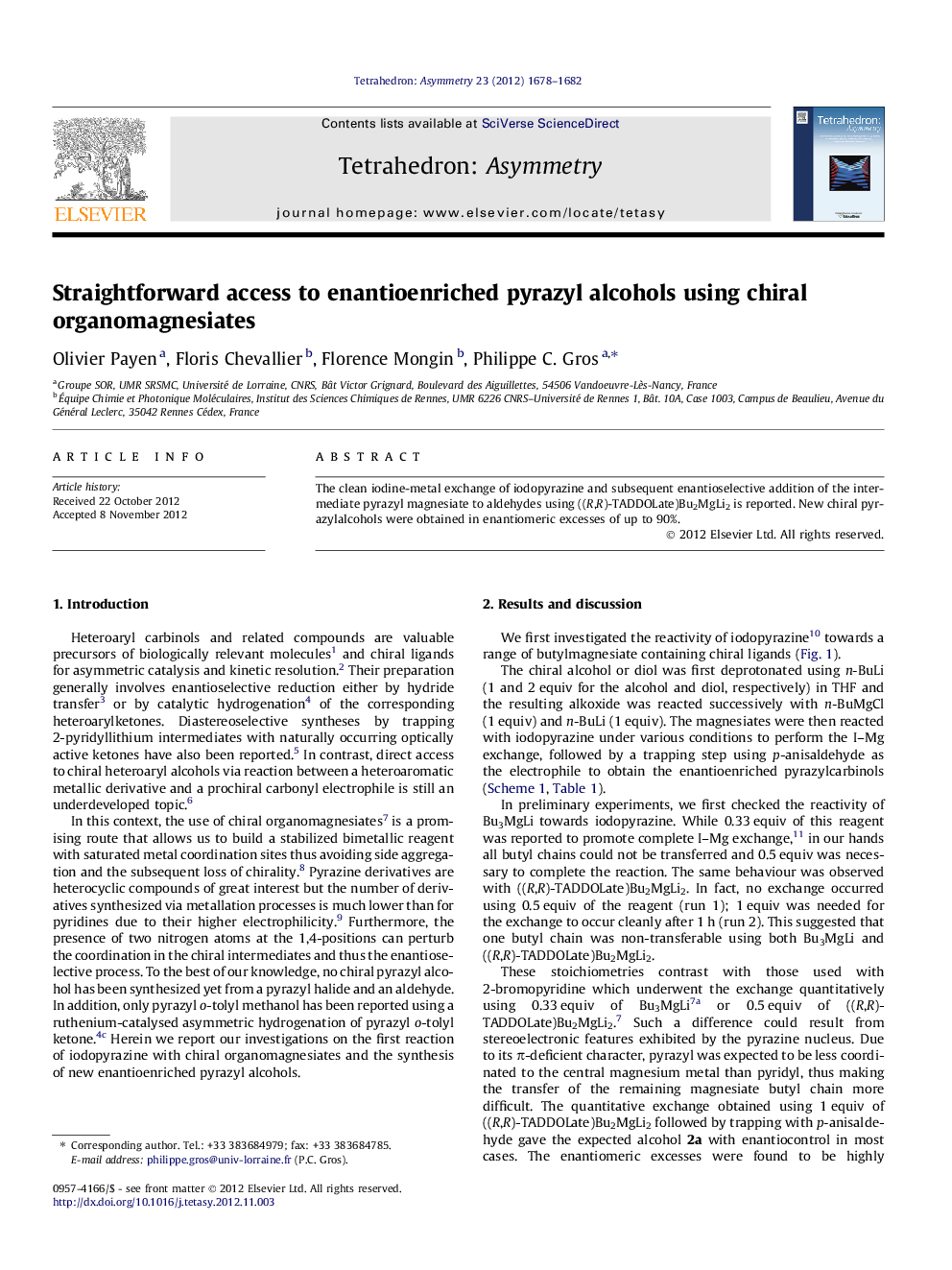
The clean iodine-metal exchange of iodopyrazine and subsequent enantioselective addition of the intermediate pyrazyl magnesiate to aldehydes using ((R,R)-TADDOLate)Bu2MgLi2 is reported. New chiral pyrazylalcohols were obtained in enantiomeric excesses of up to 90%.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-(4-Methoxyphenyl)(pyrazin-2-yl)methanolC12H12N2O2ee = 72%[α]D27.5=+43.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-(4-Dimethylaminophenyl)(pyrazin-2-yl)methanolC13H15N3Oee = 86%[α]D29=+72.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-(2-Methoxyphenyl)(pyrazin-2-yl)methanolC12H12N2O2ee = 80%[α]D29=+98.4 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-(2-Dimethylaminophenyl)(pyrazin-2-yl)methanolC12H12N3Oee = 90%[α]D23=+5.3 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-(Pyrazin-2-yl)(o-tolyl)methanolC12H12N2Oee = 64%[α]D30=+41.7 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-(2-Chlorophenyl)(pyrazin-2-yl)methanolC11H9ClN2Oee = 46%[α]D30=+61.6 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)
(S)-(2-Methoxy-1-naphthyl)(pyrazin-2-yl)methanolC16H14N2NaO2ee = 78%[α]D21=+61.0 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (S)