| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346075 | Tetrahedron: Asymmetry | 2012 | 5 Pages |
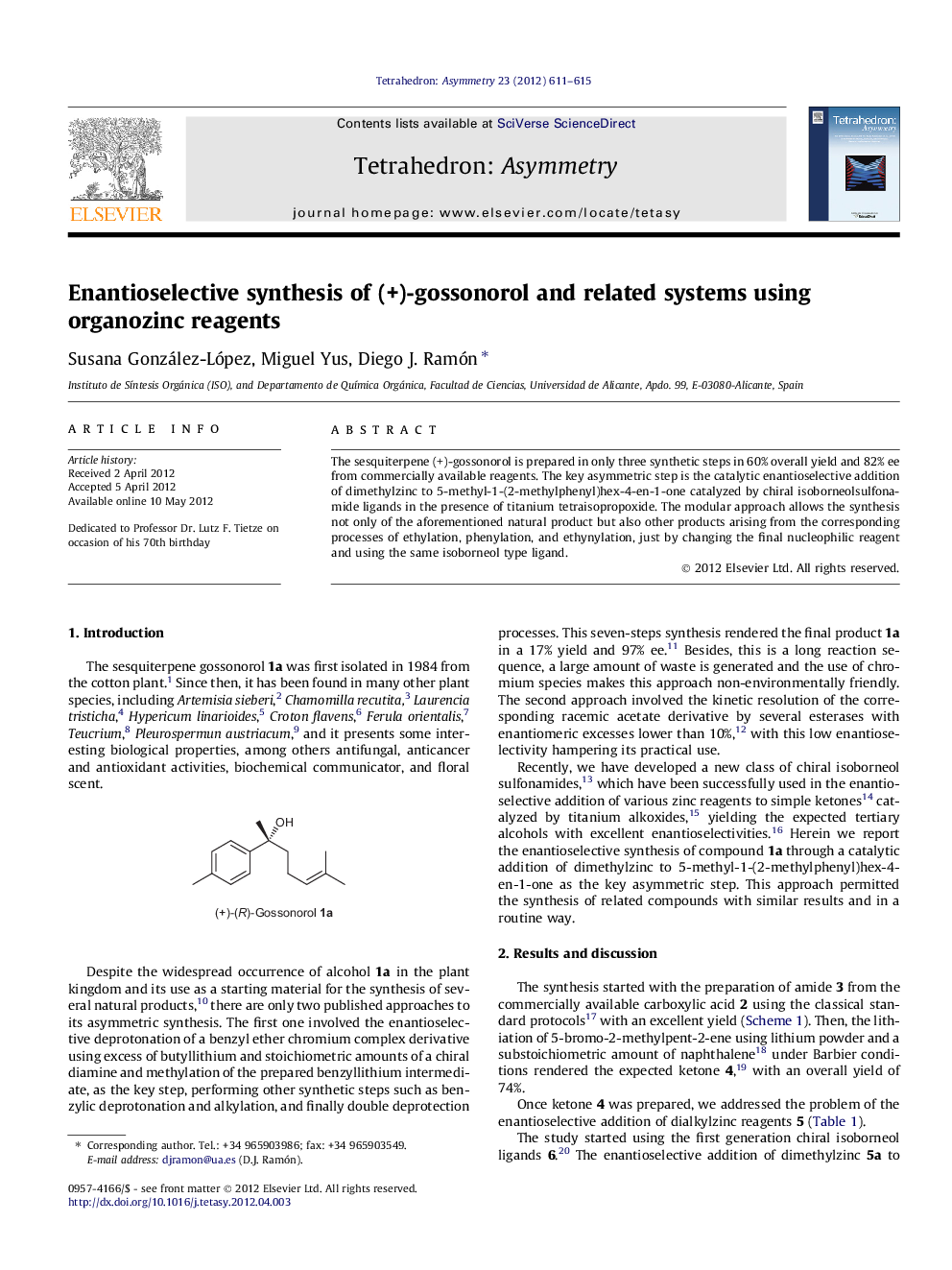
The sesquiterpene (+)-gossonorol is prepared in only three synthetic steps in 60% overall yield and 82% ee from commercially available reagents. The key asymmetric step is the catalytic enantioselective addition of dimethylzinc to 5-methyl-1-(2-methylphenyl)hex-4-en-1-one catalyzed by chiral isoborneolsulfonamide ligands in the presence of titanium tetraisopropoxide. The modular approach allows the synthesis not only of the aforementioned natural product but also other products arising from the corresponding processes of ethylation, phenylation, and ethynylation, just by changing the final nucleophilic reagent and using the same isoborneol type ligand.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(R)-6-Methyl-2-(4-methylphenyl)hept-5-en-2-olC15H22OEe = 82%[α]D20=+15.3 (c 1, CHCl3)Source of chirality: enantioselective reaction
(R)-7-Methyl-3-(4-methylphenyl)loct-6-en-3-olC16H24OEe = 64%[α]D20=+6 (c 0.3, CHCl3)Source of chirality: enantioselective reaction
(S)-5-Methyl-1-(4-methylphenyl)-1-phenyl-hex-4-en-1-olC20H24OEe = 64%[α]D20=-1.2 (c 1, CHCl3)Source of chirality: enantioselective reaction
(R)-7-Methyl-3-(4-methylphenyl)-1-phenyloct-6-en-1-yn-3-olC22H24OEe = 62%[α]D20=-3.7 (c 1, CHCl3)Source of chirality: enantioselective reaction