| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346094 | Tetrahedron: Asymmetry | 2012 | 7 Pages |
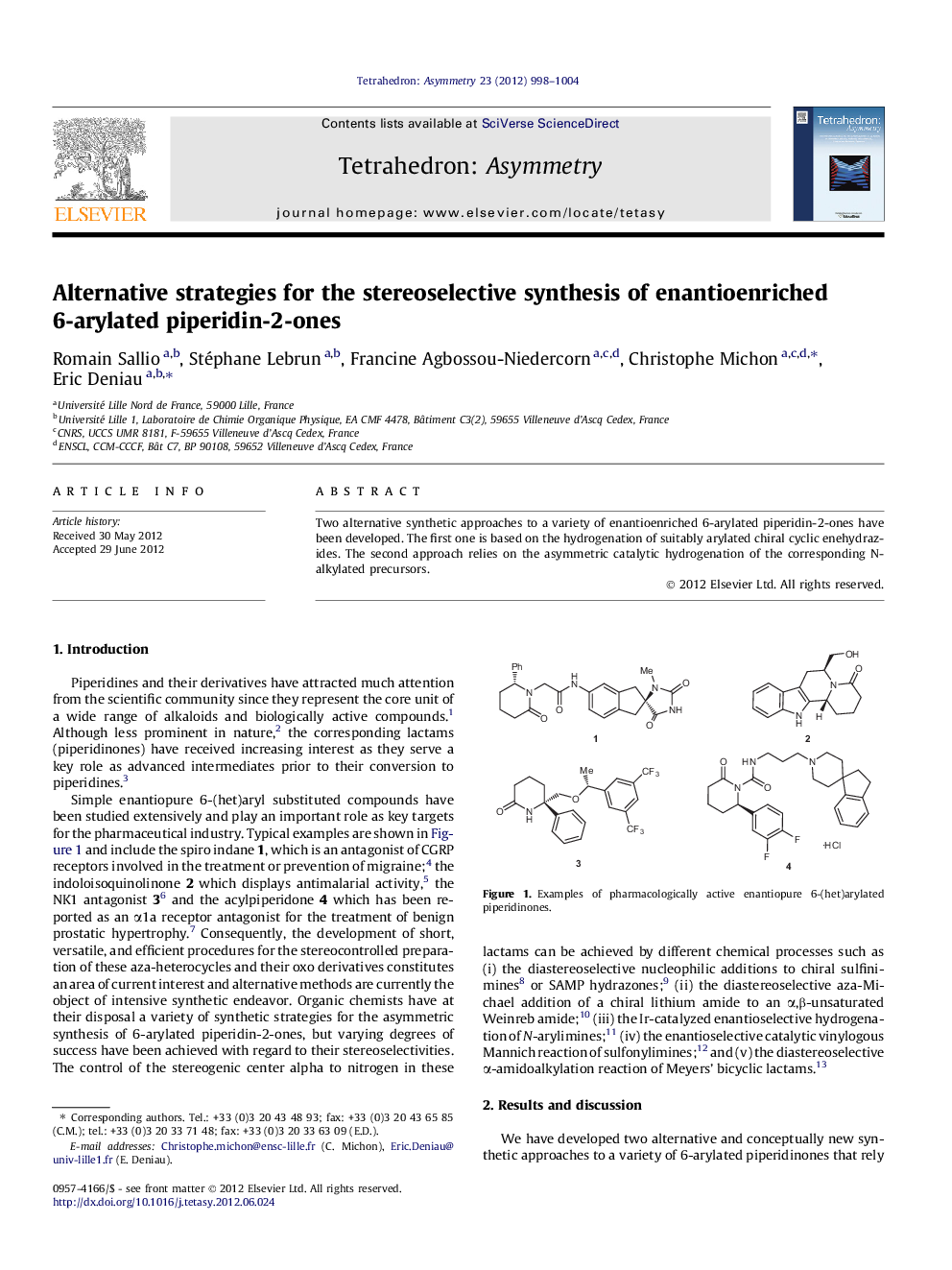
Two alternative synthetic approaches to a variety of enantioenriched 6-arylated piperidin-2-ones have been developed. The first one is based on the hydrogenation of suitably arylated chiral cyclic enehydrazides. The second approach relies on the asymmetric catalytic hydrogenation of the corresponding N-alkylated precursors.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
1-((S)-2-Methoxymethylpyrrolidin-1-yl)piperidine-2,6-dioneC11H18N2O3Ee >96%[α]D20=+3.9 (c 1.52 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S
(S)-1-(2-Methoxymethylpyrrolidin-1-yl)-6-phenyl-3,4-dihydro-1H-pyridin-2-oneC17H22N2O2Ee >96%[α]D20=-99.3 (c 1.12 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S
6-(4-Fluorophenyl)-1-((S)-2-methoxymethylpyrrolidin-1-yl)-3,4-dihydro-1H-pyridin-2-oneC17H21FN2O2Ee >96%[α]D20=-120.8 (c 1.54 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S
6-(4-Methoxyphenyl)-1-((S)-2-methoxymethylpyrrolidin-1-yl)-3,4-dihydro-1H-pyridin-2-oneC18H24N2O3Ee >96%[α]D20=-56.2 (c 2.27 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S
(S)-6-(3,4-Dimethoxyphenyl)-1-((S)-2-methoxymethylpyrrolidin-1-yl)piperidin-2-oneC19H26N2O4De >96%[α]D20=-67.3 (c 1.64 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S
6-Benzo[1,3]dioxol-5-yl-1-((S)-2-methoxymethylpyrrolidin-1-yl)-3,4-dihydro-1H-pyridin-2-oneC18H22N2O4Ee >96%[α]D20=-83.0 (c 0.53 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S
(S)-1-((S)-2-Methoxymethylpyrrolidin-1-yl)-6-phenylpiperidin-2-oneC17H24N2O2De >96%[α]D20=-56.2 (c 2.89 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S, 6S
(S)-6-(4-Fluorophenyl)-1-((S)-2-methoxymethylpyrrolidin-1-yl)piperidin-2-oneC17H23FN2O2De >96%[α]D20=-45.2 (c 1.43 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S, 6S
(S)-6-(4-Methoxyphenyl)-1-((S)-2-methoxymethylpyrrolidin-1-yl)piperidin-2-oneC18H26N2O3De >96%[α]D20=-27.4 (c 1.29 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S, 7S
(S)-6-(3,4-Dimethoxyphenyl)-1-((S)-2-methoxymethylpyrrolidin-1-yl)piperidin-2-oneC19H28N2O4Ee >96%[α]D20=-67.3 (c 1.64 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S, 6S
(S)-6-Benzo[1,3]dioxol-5-yl-1-((S)-2-methoxymethylpyrrolidin-1-yl)piperidin-2-oneC18H24N2O4De >96%[α]D20=-53.9 (c 0.49 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 2S, 6S
(S)-6-Phenylpiperidin-2-oneC11H13NOEe >96%[α]D20=-58.0 (c 0.54 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 6S
(S)-6-(3,4-Fluorophenyl)piperidin-2-oneC11H12FNOEe >96%[α]D20=-47.2 (c 0.60 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 6S
(S)-6-(3,4-Methoxyphenyl)piperidin-2-oneC12H15NO2Ee >96%[α]D20=-58.8 (c 1.90 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 6S
(S)-6-(3,4-Dimethoxyphenyl)piperidin-2-oneC13H17NO3Ee >96%[α]D20=-43.1 (c 2.16 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 6S
(S)-6-Benzo[1,3]dioxol-5-ylpiperidin-2-oneC12H13NO3Ee >96%[α]D20=-59.1 (c 0.33 in CHCl3)Source of chirality: (S)-prolineAbsolute configuration: 6S